
���������С�̫�ս�������δ�����������������������������֪�ѵĻ��ϼ���Ҫ�У�4�ۺͣ�3�ۣ����У�3�ۼ��ױ��������й��������↑ʼ������pH���£�

ij����������Ҫ�ɷ�ΪFeTiO2��������������SiO2�ȣ��ᴿTiO2���õ�����ƷFeSO4��7H2O(�̷�)�Ĺ����������£�
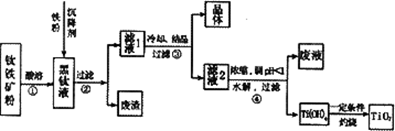
�ش��������⣺
(1)����Һ�����ɵ���Ҫ��������TiO2+��Fe2+��д����Ӧ�ٵĻ�ѧ����ʽ��
(2)����Һ�м���������ҪĿ���ǣ�________________
(3)����ܵ����ӷ���ʽ�ǣ�________
(4)�ѵ�ұ���·��ǵ�ⷨ��
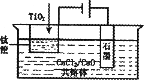
OS���������ʾ��ͼ����CaCl2/CaO��������Ϊ���ʣ����ʱ�����������ɵ�Ca��һ����ԭTiO2���ѣ�д�����������ѵ��йص缫��Ӧʽ�ͻ�ѧ����ʽ��________

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�Ͼ��и�����ѧ�����п��Ի�ѧ���� ���ͣ�ʵ����
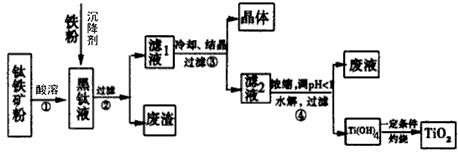
��18�֣����������С�̫�ս����������������������δ�����������������ҹ��зḻ���ѿ���Դ��
��1����ұ���ľɷ����ý������Ȼ�ԭ���Ȼ��ѡ�
��ش��������⣺
�����Ʋ⣬�ڽ���ϵ���У������� ����ϻ��á��ϲ����á����Ľ�������������ʴ����ԭ���� ��
��TiC14 ��ҵ�����ù�����̿��������TiO2�ڸ����·�Ӧ�Ƶõģ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
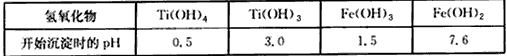
��2����֪����̬���ѣ���+4�ۺ�+3�ۣ�����+3�ۼ��ױ��������й��������↑ʼ������pH���l
��l �й��������↑ʼ������pH
|
�������� |
Ti(OH)4 |
Ti(OH)3 |
Fe(OH)3 |
Fe(OH)2 |
|
pH |
0.5 |
3.0 |
1.5 |
7.6 |
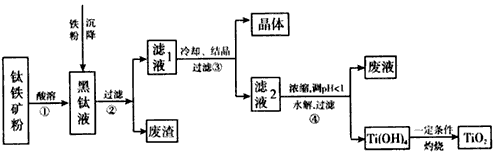
ij�����������Ҫ�ɷ�ΪFeTiO3��������������Al2O3��SiO2���ᴿTiO2���õ�����Ʒ�̷���FeSO4��7H2O���Ĺ���������ͼl��ʾ��

ͼ1 ��������ͼ
����Һ�����ɵ���Ҫ��������TiO2+��Fe2+��д����Ӧ����Ҫ�Ļ�ѧ����ʽ�� ������Fe��Ŀ���� �����������Ti(OH)4�����ӷ���ʽ�� ��
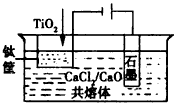
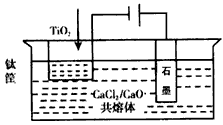
��3����ұ�����·��ǵ�ⷨ��ͼ2��һ�ֵ�����ѵĹ��գ�OS����ʾ��ͼ��CaC12/CaO������Ϊ�ʣ�������������ԭ��Ca��һ����ԭTiO2�õ��ѣ�д�������Ƶ��ѵ��йط�Ӧ����ʽ ��

��4���ҹ���ѧ�����з��ļ�������������Ĥ��ȡ�����ѣ�SOM�����գ���ͼ3���������������ȫ����Ti4+��������MCl��MF������ϵ��MΪNa��K��Ca�ȣ�����ʯīΪ����������Ϊ��������Ĥ�Ķ�����մ�Ϳ�㡣������Ĥ�����������ڵ���ʸ�����ֻ�������ӿ���ͨ����������������ͨ��H2���������缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡģ���� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡģ���� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�Ͼ��н�����ѧ������ѧ�����п��Ի�ѧ���� ���ͣ�ʵ����
��18�֣����������С�̫�ս����������������������δ�����������������ҹ��зḻ���ѿ���Դ��
��1����ұ���ľɷ����ý������Ȼ�ԭ���Ȼ��ѡ�
��ش��������⣺
�����Ʋ⣬�ڽ���ϵ���У������� ����ϻ��á��ϲ����á����Ľ�������������ʴ����ԭ���� ��
��TiC14 ��ҵ�����ù�����̿��������TiO2�ڸ����·�Ӧ�Ƶõģ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��2����֪����̬���ѣ���+4�ۺ�+3�ۣ�����+3�ۼ��ױ��������й��������↑ʼ������pH���l
��l �й��������↑ʼ������pH
| �������� | Ti(OH)4 | Ti(OH)3 | Fe(OH)3 | Fe(OH)2 |
| pH | 0.5 | 3.0 | 1.5 | 7.6 |


�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com