
����ֲ���纣���������к��д����ĵ�Ԫ�أ���Ԫ���Ե����ӵ���ʽ���ڡ�ʵ������Ӻ�������ȡ�����������ͼ��
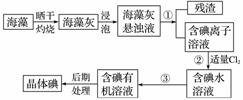
(1)ʵ���ұ��պ�������Ҫ���������е�________(����ĸ)��
a���Թ� b���ձ�
c������ d��������
e�������ż� f���ƾ���
(2)ָ����ȡ��Ĺ������йص�ʵ��������ƣ���________����__________��
(3)��ȡ��Ĺ����У���ѡ����л��Լ���________��
A���ױ����ƾ� B�����Ȼ�̼����
C�����͡����� D�����͡�����
(4)Ϊʹ������е�����ת��Ϊ����л���Һ��ʵ�������ձ���������������ƿ���ƾ��ơ����ܡ�Բ����ƿ��ʯ�����Լ���Ҫ�ļг���������Ʒ����ȱ�ٵIJ���������______��______________��

(5)С����CCl4��ȡ��ˮ�еĵ⣬����ͼ�ķ�Һ©���У��²�Һ���______ɫ�����Ǵ�Һ©��������ȴδ��Һ�����£�ԭ�������
________________________________________________________________________��
(6)�Ӻ�����л���Һ����ȡ��ͻ����л��ܼ������뾭������ָ������ʵ��װ��ͼ�еĴ���֮����

��________________________________________________________________________��
��________________________________________________________________________��
��________________________________________________________________________��
(7)���������������ʱ��ʹ��ˮԡ���ȵ�ԭ����_______________________________��
���̬����________��ۼ���
 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Na2CO3����ȡ�������ۺ�ʵ��̽��
(1)�ҹ���ѧ�Һ�°�ĸ����Ĵ����������գ����������̿ɼ�Ҫ��ʾ���£�

��������ʳ��ˮ������ͨ��������NH3��CO2(���)��������ͨCO2��ͨNH3��ԭ����________________________________________________________________________��
�ڴ���������ͼ��֪����ѭ�����õ�������________��
��д���������С�����¯�з�Ӧ�Ļ�ѧ����ʽ___________________________��
(2)�����CO2��50 mL 2 mol·L��1 NaOH��Һ��ȡ50 mL 1 mol·L��1 Na2CO3��Һ��
��д����Ҫ��ʵ�鲽�裺_________________________________________________��
��д���йط�Ӧ�Ļ�ѧ����ʽ��______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��Z��W��R��5�ֶ�����Ԫ�أ���ԭ��������������X�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�Yԭ�������������Ǵ�����������3����Z��W��R����ͬһ���ڣ�R��Y����ͬһ�壬Z��Wԭ�ӵĺ��������֮����Y��Rԭ�ӵĺ��������֮����ȡ�����˵����ȷ����(����)
A��Ԫ��Y��Z��W������ͬ���Ӳ�ṹ�����ӣ���뾶��������
B��Ԫ��X������Ԫ��Y�γɻ�����X2Y2
C��Ԫ��Y��R�ֱ���Ԫ��X�γɵĻ���������ȶ��ԣ�XmY>XmR
D��Ԫ��W��R������������ˮ���ﶼ��ǿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з������ʵķ����У����������ʵķе����(����)
A������ B����ȡ C���ؽᾧ D������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���г��ӷ����������(����)
| ѡ�� | ���ᴿ������ | ���� | �����Լ� | ���ӷ��� |
| A | CO(g) | CO2(g) | NaOH��Һ��ŨH2SO4 | ϴ�� |
| B | NH4Cl(aq) | Fe3��(aq) | NaOH��Һ | ���� |
| C | Cl2(g) | HCl(g) | ����ʳ��ˮ��ŨH2SO4 | ϴ�� |
| D | Na2CO3(s) | NaHCO3(s) | — | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���³��ӷ���(������Ϊ��������)�У��������(����)
A��NaCl(Na2CO3)���ܽ⣬�����������ᣬ�����ᾧ
B��Na2SO4(NH4Cl)�����ȡ�����
C��CO2(CO)��ͨ������CuO��ĩ
D��H2(NH3)��ͨ��ʢŨH2SO4��ϴ��ƿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
1919�꣬Langmuir����ȵ���ԭ����ԭ������ͬ������������ͬ�ķ��ӣ�����Ϊ�ȵ����塣�ȵ�����Ľṹ���ơ��������������
(1)��������ԭ�������ɵ�2����Ԫ����ɵĹ��۷����У���Ϊ�ȵ��������________��________��________��
________________________��
(2)�˺ȵ���ԭ����������չ�����磬�ɶ�����Ԫ����ɵ�����ֻҪ��ԭ������ͬ����ԭ������������֮����ͬ��Ҳ�ɻ���Ϊ�ȵ����壬����Ҳ�������ƵĽṹ�������ڶ�����Ԫ����ɵ������У���NO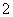 ��Ϊ�ȵ�����ķ�����________��________��
��Ϊ�ȵ�����ķ�����________��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͭ�����백ˮ����������ⶼ���ܷ�Ӧ�������백ˮ��������Ļ����Һ��Ӧ����ԭ����______________________________________________��
��Ӧ�Ļ�ѧ����ʽΪ_________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com