
ij �о�С����ȡ��������Ⱦ��ˮԴ���������·���������������ʵ����Ϣ������һ������Ⱦ��ˮԴ����A��B�������ʣ�һ������C��D�������ʣ�һ������E���ʣ�A��B��C��D��EΪ���ֳ�������������±��е������γɣ�
�о�С����ȡ��������Ⱦ��ˮԴ���������·���������������ʵ����Ϣ������һ������Ⱦ��ˮԴ����A��B�������ʣ�һ������C��D�������ʣ�һ������E���ʣ�A��B��C��D��EΪ���ֳ�������������±��е������γɣ�
| ������ | K����Na����Cu2����Al3�� |
| ������ | SO |
Ϊ�˼�������������ֱ��������ʵ�飬������
�ٽ���������ˮ��DΪ��ɫ��Һ��������Ϊ��ɫ��Һ��
�ڽ�E��Һ���뵽C��Һ�г��ְ�ɫ�����������μӣ������ܽ⣻
�۽�����ɫ��Ӧ��ֻ��B��CΪ��ɫ(����ɫ�ܲ���)��
���ڸ���Һ�м������ᱵ��Һ���ټ������ϡ���ᣬA�зų���ɫ���壬C��D�в�����ɫ������
�ݽ�B��D����Һ��ϣ�δ���������������ɡ�
��������ʵ����գ�
(1)д��B��D�Ļ�ѧʽ��B________��D________��
(2)д��D��E������Ӧ�����ӷ���ʽ��____________________________________��
(3)����1 mol A����Һ�뺬1 mol E����Һ��Ӧ�����ɣ����õ�һ�ֻ�����û�����Ϊ__________��
(4)C��������ˮ���������ӷ���ʽ��ʾ�侻ˮԭ����______ ______________________��
______________________��
�𰸡�(1)KNO3��CuSO4
(2)Cu2����2OH��===Cu(OH)2��
(3)Na2CO3
(4)Al3����3H2OAl(OH)3(����)��3H��
����������ʵ��ٿ�֪��D�к���Cu2��������ʵ��ڿ�֪C�к���Al3����E������KOH��NaOH���ٸ��ݢۿ�֪��ֻ��B��C�к���K������EΪNaOH������ʵ��ܿ�֪A�к���HCO ����AΪNaHCO3��C��D�к���SO
����AΪNaHCO3��C��D�к���SO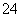 ����DΪCuSO4��CΪKAl(SO4)2�������ж�BΪKNO3�������ʵ�����NaHCO3��NaOH��Ӧ����Na2CO3��H2O��KAl(SO4)2��Һ�е�Al3��ˮ�����ɾ����������õ�Al(O
����DΪCuSO4��CΪKAl(SO4)2�������ж�BΪKNO3�������ʵ�����NaHCO3��NaOH��Ӧ����Na2CO3��H2O��KAl(SO4)2��Һ�е�Al3��ˮ�����ɾ����������õ�Al(O H)3�������ˮ��
H)3�������ˮ��


 ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ���� (����)
A������������Ⱦ����Էֽ⣬�������NH3
B������������ֽⱬը��ʵ���ҳ������Ȼ�����������ƵĻ�����Ʊ�����
C����ʢ�����������[(NH4)2Fe(SO4)2]��Һ���Թ��У��μ�����NaOH��Һ�����Թܿ���ʪ��ĺ�ɫʯ����ֽ���飬��ֽ����
D����ζ�������ˮ����ˮ��Һ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и��������У��������ͼʾ������ͨ����������һ��ת�������ֻ��(����)
| ��� | X | Y | Z | W |
|
| �� | Cu | CuSO4 | Cu(OH)2 | CuO | |
| �� | Na | NaOH | Na2CO3 | NaCl | |
| �� | Cl2 | Ca(ClO)2 | HClO | HCl | |
| �� | Fe | FeCl3 | FeCl2 | Fe(OH)2 |
A.�٢ڢ� B���٢ۢ�
C���ڢ� D���٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ѣ�CH3OCH3���ͼ״���CH3OH�����Ǹ�Ч�����Դ����ҵ������ú���������ˮú�����ϳɼ״��Ͷ����ѡ��ش��������⣺
(1)�Ʊ����������һ����Ӧ��Al2O3���״���ˮ�ϳɣ���Ӧ����ʽΪ ��
(2)��֪��CO(g)+2H2(g)=CH3OH (g) ��H= ��90.1kJ��mol-1
CO(g)��ȼ������282.8 kJ��mol-1��H2��ȼ������285.8 kJ��mol-1
д����ʾCH3OH (g) ȼ���ȵ��Ȼ�ѧ��Ӧ����ʽ ��
��3��������ֱ��ȼ�ϵ�رȼ״�ֱ��ȼ�ϵ�ظ���Ч���������Ķ����Ѻͼ״���ȫ�ŵ�ת�Ƶ��ӵ����ʵ���֮���� ���ö�����ֱ��ȼ�ϵ�ص����������ʳ��ˮ��������9.2g������ʱ������������������������Ϊ L��������£�
��4���ںϳ��а���ˮú��������Ӧ��CO(g)+H2O(g)  CO2(g)+H2(g)�������ʵ�����CO(g)��H2O(g)�����ܱ������з�Ӧ��ƽ��ʱ��ý�����±���
CO2(g)+H2(g)�������ʵ�����CO(g)��H2O(g)�����ܱ������з�Ӧ��ƽ��ʱ��ý�����±���
| �¶� | 260�� | 280�� | 295�� | 310�� |
| COת���� | 89% | 80% | 75% | 60% |
�������COת�������¶ȱ仯�Ĺ�ϵ ��
����ʽ����280��ʱƽ�ⳣ�� ��
����ƽ����ϵ�У����H2��ѹǿռ��ѹ��30%��Ҫʹ��ϵ��COת���ʴﵽ70%��Ӧ��ʹ�¶� ������ߡ��������͡��������䡱��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ������֮���ת����ϵ����֪D��E��Z����ѧ��ѧ�����ĵ��ʣ��������ǻ����Z��Y���ȼ� ��ҵ�IJ�Ʒ��DԪ�ص�ԭ����������������Ӳ�����ȣ���D�������ο�����ˮ����EΪ�ճ�������Ӧ����㷺�Ľ���������Ӧ���⣬������Ӧ����ˮ��Һ�н��С���ش��������⡣
��ҵ�IJ�Ʒ��DԪ�ص�ԭ����������������Ӳ�����ȣ���D�������ο�����ˮ����EΪ�ճ�������Ӧ����㷺�Ľ���������Ӧ���⣬������Ӧ����ˮ��Һ�н��С���ش��������⡣

(1)д���������ʵĻ�ѧʽ��B____________��G_____________________________��Y______________��
(2)�ڵ�ƹ�ҵ�У�����E��Ϊ���ƽ�����ͭΪ�Ʋ��������E��__________������д���ڴ˵缫�Ϸ����ĵ缫��Ӧʽ��
________________________________________________________________________��
(3)д����Ӧ�ٵĻ�ѧ����ʽ______________________________________________��
(4)A��Һ��NaOH��Һ��Ͽ��γɳ�����ij�¶��´˳�����Ksp��2.097��10��39����
0.01 mol·L��1��A��Һ��0.001 mol·L��1��NaOH��Һ�������ϣ�����Ϊ�ܷ��γɳ���________(��ܡ����ܡ�)����ͨ������˵��___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ɰ����ӵ�����(NA)��һ��ˮ���ӵ�����(mˮ)��һ��ˮ���ӵ����(Vˮ)����ȷ������������________��
��1Ħ��ˮ����������1Ħ��ˮ��������������1Ħ��ˮ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��a g�������̷�ĩ����c L b mol·L��1��Ũ�����м�����ȫ�ܽ⣬��Ӧ��ת�Ƶ���d������NAΪ�����ӵ�������ֵ������������ȷ���� (����)
A�������ռ�������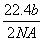 L
L
B����Ӧ����Һ�е�Cl����ĿΪ
C��NA�ɱ�ʾΪ
D����Ӧ����Һ�е�H����ĿΪbc��2d
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����
A����֬��һ�������ܷ���ˮ�ⷴӦ,���ڸ߷���
B�������ʶ��ǽṹ���ӵĸ߷��ӻ���������ж�����C��H��O��N����Ԫ��
C���ޡ��顢��ë���ϳ���ά��ȫȼ�ն�ֻ����CO2��H2O
D��������ɻ���ʼ�ɡ��еġ��ᡱ��Ҫ���л�ϩ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com