


 ĆĻ½ØĘ½Š”ѧ¹ö¶Æ²āŹŌĻµĮŠ“š°ø
ĆĻ½ØĘ½Š”ѧ¹ö¶Æ²āŹŌĻµĮŠ“š°ø »ĘøŌĢģĢģĮ·æŚĖćĢāæØĻµĮŠ“š°ø
»ĘøŌĢģĢģĮ·æŚĖćĢāæØĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Cl”„ | B£®SO32”„ | C£®HCO3”„ | D£®Al3+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
A£®x=10Ź±£¬ČÜŅŗÖŠÓŠ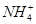 ”¢ ”¢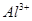 ”¢ ”¢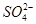 £¬ĒŅ £¬ĒŅ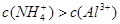 |
B£®x=10Ź±£¬ČÜŅŗÖŠÓŠ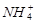 ”¢ ”¢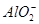 ”¢ ”¢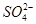 £¬ĒŅ £¬ĒŅ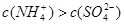 |
C£®x=30Ź±£¬ČÜŅŗÖŠÓŠ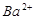 ”¢ ”¢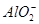 ”¢ ”¢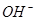 £¬ĒŅ £¬ĒŅ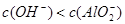 |
D£®x=30Ź±£¬ČÜŅŗÖŠÓŠ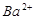 ”¢ ”¢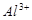 ”¢ ”¢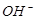 £¬ĒŅ £¬ĒŅ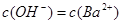 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
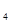 )2
)2 ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®MgCl2 | B£®H2SO4 | C£®NH4HCO3 | D£®Mg£ØHCO3£©2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
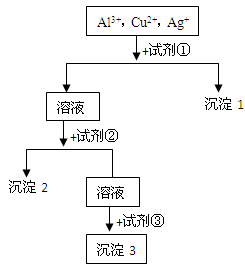
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Mg2+£¬NH4+£¬Cl-£¬SO42- | B£®Na+£¬Cu2+£¬CO32-£¬NO3- |
| C£®Ba2+£¬K+£¬SO32-£¬Cl-£¬ | D£®Na+£¬Fe2+£¬SO42-£¬NO3- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 3£©ĮķČ”²æ·ÖČÜŅŗ£¬¼ÓČėÉŁĮ潚ŹōĶ·Ū£¬Ķ·ŪČܽā£¬ĒŅÓŠĘųÅŻĆ°³ö£¬ŌņČÜŅŗÖŠæĻ¶Ø“ęŌŚµÄŅõĄė×ÓŹĒ ”£
3£©ĮķČ”²æ·ÖČÜŅŗ£¬¼ÓČėÉŁĮ潚ŹōĶ·Ū£¬Ķ·ŪČܽā£¬ĒŅÓŠĘųÅŻĆ°³ö£¬ŌņČÜŅŗÖŠæĻ¶Ø“ęŌŚµÄŅõĄė×ÓŹĒ ”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
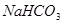 £»¢Ś
£»¢Ś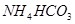 £»¢Ū
£»¢Ū £»¢ÜHF£»¢Ż
£»¢ÜHF£»¢Ż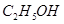 £»¢Ž
£»¢Ž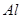 £»¢ßŹ³ŃĪĖ®£»¢ą
£»¢ßŹ³ŃĪĖ®£»¢ą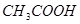
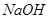 ČÜŅŗ·“Ó¦µÄŹĒ_____________”£
ČÜŅŗ·“Ó¦µÄŹĒ_____________”£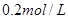 µÄijĖįČÜŅŗ£¬æÉÄÜĪŖ“×Ėį”¢ŃĪĖį”¢ĮņĖįÖŠµÄŅ»ÖÖ£¬ĪŖĮĖČ·¶ØøĆĖįČÜŅŗµÄ×é³É½ųŠŠŹµŃé£ŗČ”
µÄijĖįČÜŅŗ£¬æÉÄÜĪŖ“×Ėį”¢ŃĪĖį”¢ĮņĖįÖŠµÄŅ»ÖÖ£¬ĪŖĮĖČ·¶ØøĆĖįČÜŅŗµÄ×é³É½ųŠŠŹµŃé£ŗČ”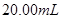 øĆĖį
øĆĖį ČÜŅŗ£¬ÖšµĪ¼ÓČė
ČÜŅŗ£¬ÖšµĪ¼ÓČė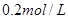 µÄĒāŃõ»ÆÄĘČÜŅŗ£¬Ē”ŗĆ·“Ó¦ĶźČ«Ź±ĖłŠč¼īŅŗĢå»żĪŖ
µÄĒāŃõ»ÆÄĘČÜŅŗ£¬Ē”ŗĆ·“Ó¦ĶźČ«Ź±ĖłŠč¼īŅŗĢå»żĪŖ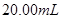 £¬ĒėÓĆ»ÆѧÓĆÓļ»Ų“š£ŗ
£¬ĒėÓĆ»ÆѧÓĆÓļ»Ų“š£ŗ _______________”£
_______________”£| ŃōĄė×Ó | 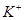 ”¢ ”¢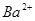 ”¢ ”¢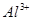 ”¢ ”¢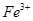 |
| ŅõĄė×Ó | 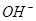 ”¢ ”¢ ”¢ ”¢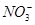 ”¢ ”¢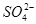 |
 ČēĻĀ£ŗ
ČēĻĀ£ŗ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com