
̼����ĺ���Ӱ��������ܡ�̼��������һ�ֲⶨ�����ǽ������е�̼����ת��Ϊ���壬���ò�̼������װ�ý��вⶨ��
(1)����װ��A���ڸ����½�x g�����е�̼����ת��ΪCO2��SO2��
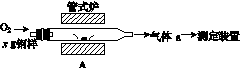
������a�ijɷ���______��
��������������FeS��ʽ���ڣ�A�з�Ӧ��
3FeS��5O2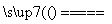 1________��3________��
1________��3________��
(2)������aͨ�����װ����(��ͼ)�����õζ����ⶨ��ĺ�����
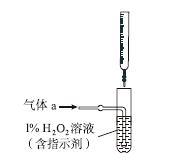
��H2O2����SO2�Ļ�ѧ����ʽ��__________________��
����NaOH��Һ�ζ����ɵ�H2SO4������z mL NaOH��Һ��������1 mL NaOH��Һ�൱���������Ϊy g����ø������������������________��
(3)������aͨ���̼װ����(��ͼ)�������������ⶨ̼�ĺ�����

������aͨ��B��C��Ŀ����________________________________________��
�ڼ��������̼������������Ӧ������������_______________________________________________________��
(1)��O2��SO2��CO2����Fe3O4��SO2��
(2)��H2O2��SO2===H2SO4����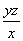 ��
��
(3)���ų�SO2��CO2�ⶨ�ĸ��š�������CO2ǰ��������ƿ������
[����] (1)�ٸ����������գ�̼����ת��Ϊ������̼�Ͷ���������������ɷ�ΪCO2��SO2��O2��������Ԫ�صĴ�����ʽΪFeS�����ݸ����Ļ�ѧ��������3���������ΪSO2������������غ�ȷ��1���������ΪFe3O4����ѧ����ʽΪ3FeS��5O2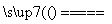 Fe3O4��3SO2��
Fe3O4��3SO2��
(2)��H2O2��SO2��Ӧ�Ļ�ѧ����ʽΪH2O2�� SO2=== H2SO4���ڸ�������1 mL������������Һ�൱������y g��������z mL������������Һ�൱�ں�����Ϊzy g�������������Ϊ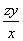 ��
��
(3)�����мȺ��ж��������ֺ��ж�����̼���ⶨ������̼ǰ�����ȥ������������ţ�����B��Cװ�����ڳ�ȥ������̼�еĶ������ⶨ̼�ĺ������ⶨ������̼�����������Ҫ�ⶨ�����������ն�����̼װ��(������̼����ƿ)ǰ�������(������ֵΪ������̼������)��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ҩ�ﰢĪ������ɱ�������ϸ����ֳ�����ĺϳ�·�����£�
 |
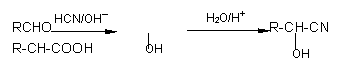 |
��֪��
A��ʹ���Ȼ�����Һ��ɫ��
���������գ�
��1��д��A�Ľṹ��ʽ��_______________��CH3I������_____________________��
��2��д����Ӧ���ͣ���Ӧ��____________����Ӧ��______________��
��3��G�й����ŵ�����_______________��
��4����Ӧ�ܵĻ�ѧ����ʽ______________________________________��
H��һ��ͬ���칹���һ�ȴ���ĽṹΪ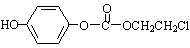 ����������NaOHˮ��Һ�м��ȷ�Ӧʱ�Ļ�ѧ����ʽΪ������������������������������������������������
����������NaOHˮ��Һ�м��ȷ�Ӧʱ�Ļ�ѧ����ʽΪ������������������������������������������������
��5����д����ͬʱ������������H���Ե�ͬ���칹����������������������������������
a�������Ȼ�����Һ������ɫ��Ӧ������b������̼�����Ʒ�Ӧ������ɫ����
c��ȡ0.1mol�л�����������Na��Ӧ�ܲ���3.36L������£�����
d�������ϵ�һ�ȴ���ֻ�����֣�����������ԭ��������3
e�������к��м�
��6��������CH3CH��CH2Ϊԭ���Ʊ� �ĺϳ�·������ͼ�����Լ����ã���
�ĺϳ�·������ͼ�����Լ����ã���
�ϳ�·������ͼ�����£�
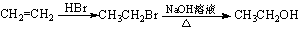
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ���ʵ�ּ���Ŀ�ĵ���(����)
A����KOH��Һ����SO3(g)��SO2
B����ʪ��⻯�ص�����ֽ����Br2(g)��NO2
C����CO2����NaAlO2��Һ��CH3COONa��Һ
D����BaCl2��Һ����AgNO3��Һ��K2SO4��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ�����Ʊ���������ʱ�����÷�����ȷ����(����)
A��������ʱ����Na2O2��H2O2����Ӧ���ѡ����ͬ�����巢��װ��
B��������ʱ���ñ���NaHCO3��Һ��Ũ���Ά������
C������ϩʱ������ˮ���������ſ������ռ�����
D���ƶ�������ʱ����ˮ��NaOH��Һ����β��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ҷ����Ԫ�صļ���ɾ������ĸ�������ɣ���������ѡ�õ�ʵ����Ʒ���ܶ��õ�����(����)
A������Ҷ���ջһ���ѡ�â١��ں͢�
B����Ũ�����ܽ��Ҷ��������ˮϡ�ͣ�ѡ�âܡ��͢�
C�����˵õ���Һ��ѡ�âܡ��ݺ͢�
D��������Һ�е�Fe3����ѡ�âۡ���͢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ�ϳ����ú����ˮ����Na2S2O3��5H2O��ʵ���ҿ�������װ��(��ȥ���ּг�����)ģ���������̡�
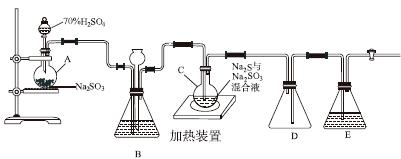
��ƿC�з�����Ӧ���£�
Na2S(aq)��H2O(l)��SO2(g)===Na2SO3(aq)��H2S(aq)��(��)
2H2S(aq)��SO2(g)===3S(s)��2H2O(l)��(��)
S(s)��Na2SO3(aq) Na2S2O3(aq)��(��)
Na2S2O3(aq)��(��)
(1)������װ��ɺر����˻�������װ��B�еij���©����ע��Һ�����γ�һ��Һ������________________��������װ�����������á�װ��D��������__________��װ��E��Ϊ________��Һ��
(2)Ϊ��߲�Ʒ���ȣ�Ӧʹ��ƿC��Na2S��Na2SO3ǡ����ȫ��Ӧ������ƿC��Na2S��Na2SO3���ʵ���֮��Ϊ________��
(3)װ��B������֮һ�ǹ۲�SO2���������ʣ����е�Һ�����ѡ��________��
a������ˮ b������Na2SO3��Һ
c������NaHSO3��Һ d������NaHCO3��Һ
ʵ���У�ΪʹSO2����������ƿC�����õIJ�����__________________________����֪��Ӧ(��)��Խ���������ƿC�з�Ӧ�ﵽ�յ��������__________________����Ӧ���ڿ��þƾ����ʵ�������ƿA��ʵ�����þƾ��Ƽ���ʱ����ʹ��ʯ��������������________��
a���ձ� b��������
c���Թ� d����ƿ
(4)��Ӧ��ֹ����ƿC�е���Һ������Ũ������ȴ�ᾧ��������Na2S2O3��5H2O�����п��ܺ���Na2SO3��Na2SO4�����ʡ����������Լ����ʵ�飬����Ʒ���Ƿ����Na2SO4����Ҫ˵��ʵ�����������ͽ��ۣ�________________________________________��
��֪Na2S2O3��5H2O�����ֽ⣺S2O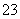 ��2H��===S����SO2����H2O
��2H��===S����SO2����H2O
��ѡ����Լ���ϡ���ᡢϡ���ᡢϡ���ᡢBaCl2��Һ��AgNO3��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ���ҴӺ����Һ(��H2O�⣬����CCl4��I2��I����)�л��յ⣬��ʵ��������£�

(1)���Һ�м����Թ�����Na2SO3��Һ������Һ�е�I2��ԭΪI���������ӷ���ʽΪ__________________���ò�����I2��ԭΪI����Ŀ����______________________��
(2)����X������Ϊ________��
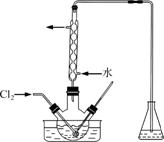
(3)����ʱ����������ƿ�н���I����ˮ��Һ���������pHԼΪ2������ͨ��Cl2����40 �����ҷ�Ӧ(ʵ��װ����ͼ��ʾ)��
ʵ������ڽϵ��¶��½��е�ԭ����______________����ƿ��ʢ�ŵ���ҺΪ________��
(4)��֪��5SO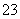 ��2IO
��2IO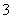 ��2H��===I2��5SO
��2H��===I2��5SO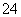 ��H2O
��H2O
ij�����ˮ(pHԼΪ8)��һ������I2�����ܴ���I����IO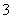 �е�һ�ֻ����֡��벹���������麬���ˮ���Ƿ���I����IO
�е�һ�ֻ����֡��벹���������麬���ˮ���Ƿ���I����IO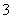 ��ʵ�鷽����ȡ���������ˮ��CCl4�����ȡ����Һ��ֱ��ˮ���õ�����Һ���鲻���еⵥ�ʴ��ڣ�________________________________________________________________________
��ʵ�鷽����ȡ���������ˮ��CCl4�����ȡ����Һ��ֱ��ˮ���õ�����Һ���鲻���еⵥ�ʴ��ڣ�________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
ʵ���пɹ�ѡ����Լ���ϡ���ᡢ������Һ��FeCl3��Һ��Na2SO3��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
MnO2��һЩ���ʻ���;��ͼ������˵����ȷ���� ( )
A���١��ڡ���������Ӧ��Mn02����������
B������Mn02��2 L 10 mol��l�� HCl���ȣ�������5 mol C12
C����Ӧ��������1 mol Al203����Ӧ������ת��12 mol���ӡ�
D����Ӧ����K2CO3��KNO3�Ļ�ѧ��������Ϊ1
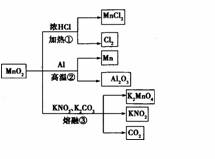
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ֲ��ӷ��͵ijɷ�֮һ�����Ľṹ��ʽΪ��HO�� ��CH2CH=CH2�����������в���ȷ����( )
��CH2CH=CH2�����������в���ȷ����( )
A��1mol������������4molH2������Ӧ
B��1mol������������4mol�巢����Ӧ
C�������ӿ����ȩ������Ӧ�����ɾۺ���
D����������ˮ�е��ܽ��С�ڱ�����ˮ�е��ܽ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com