
һ������Ķ�����̼�����ΪV mL������������Һ��ȫ���պ����鷢����Һ�е����������Ѿ���ȫ��Ӧ����������Ӧ���Һ���Ϊ���ȷݡ�
��1����ͬѧ�����е�һ�ݼ��������ij���ʯ��ˮ���õ�����������ΪM g���ݴˣ��ܷ�ȷ�����ͨ���CO2�ڱ�״���µ����������ܣ����г�����ʽ��������ܣ���������ĺ�������д�����ܡ���
��_________________________________________________________________________��
�ܷ�ݴ�ȷ������������Һ��Ũ�ȣ�����ܣ����г�����ʽ��������ܣ���������ĺ�������д�����ܡ���
��___________________________________________________________________________��
��2����ͬѧ��ڶ�����Һ�еμ�������CaCl2��Һ�����յõ��������N g���ݴ��ж�M��N����Դ�С��ϵ�ǣ�__________________________________________________��
��1���� 2��22.4 L��mol-1��M g/100 g��mol-1(��0.448M L) ����
��2��M��N
��1��������֪��CO2��NaOHǡ����ȫ��Ӧ�����ܷ�ӦΪ��CO2+2NaOH====Na2CO3+H2O CO2+NaOH====NaHCO3
��Ӧ��ĵ�һ����Һ�м�����������ʯ��ˮʱ�����ܷ�ӦΪ��Na2CO3+Ca(OH)2====CaCO3��+2NaOH��NaHCO3+Ca(OH)2====CaCO3��+H2O+NaOH�����������ĸ���Ӧ����ʽ�ɵù�ϵʽ��CO2��CaCO3����
��һ�ݣ�n(CO2)=n(CaCO3)=![]() �����ͨ���CO2���Ϊ��V��CO2��=2��22.4 L��mol-1��M g/100 g��mol-1=0.448M L��
�����ͨ���CO2���Ϊ��V��CO2��=2��22.4 L��mol-1��M g/100 g��mol-1=0.448M L��
���ڲ�֪��NaOH�����ʵ������ʲ���ȷ��NaOH��Һ��Ũ�ȡ�
��2����ڶ�����Һ�еμ�������CaCl2��Һʱ��������Һ�п���ֻ��Na2CO3��Ҳ������Na2CO3��NaHCO3�Ļ����Һ����ֻ������ӦCaCl2+Na2CO3====CaCO3��+2NaCl������M��N��


 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��ͬѧ�����е�һ�ݼ��������ij���ʯ��ˮ���õ�����������ΪM g���ݴˣ��ܷ�ȷ�����ͨ���CO2�ڱ�״���µ����������ܣ����г�����ʽ��������ܣ���������ĺ�������д�����ܡ���
��_________________________________________________________________��
�ܷ�ݴ�ȷ������������Һ��Ũ�ȣ�����ܣ����г�����ʽ��������ܣ���������ĺ�������д�����ܡ���
��_________________________________________________________________��
(2)��ͬѧ��ڶ�����Һ�еμ�������CaCl2��Һ�����յõ��������N g���ݴ��ж�M��N����Դ�С��ϵ�ǣ�__________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡΫ���и���3�µ�һ��ģ�⿼�Ի�ѧ�Ծ����������� ���ͣ������
�״���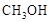 ������Ҫ����Դ���ʣ��о��״�������Ҫ���塣
������Ҫ����Դ���ʣ��о��״�������Ҫ���塣
��1�����ù�ҵ�����е�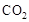 ����ȡ�״����䷴ӦΪ��
����ȡ�״����䷴ӦΪ��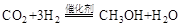
���³�ѹ����֪���з�Ӧ�������仯��ͼ��ʾ��
д���ɶ�����̼�������Ʊ��״����Ȼ�ѧ����ʽ��
��
��2��Ϊ��״�ȼ�ϵ������ʣ���ѧ�ҷ�����һ��ȼ�ϵ�أ���ص�һ���缫ͨ���������һ���缫ͨ��״����壬������Dz�����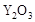 ��
�� 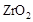 ���壬�ڸ��������ܴ���
���壬�ڸ��������ܴ���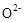 ���ӡ���ع���ʱ������ӦΪ ��
���ӡ���ع���ʱ������ӦΪ ��
���Ըõ��Ϊ��Դ����ʯī���缫���100mL�����������ӵ���Һ��
���һ��ʱ��������ռ�����ͬ�������ͬ������������ʱ��������Һ����ı仯���缫������ܴ��ڵ��ܽ������������ռ������������ʵ���Ϊ mol��
��3���״���ˮ�ʻ����һ������Ⱦ����һ�ֵ绯ѧ��������������Ⱦ����ԭ���ǣ�ͨ���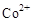 ������
������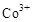 ��Ȼ����
��Ȼ����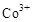 ����������ˮ�еļ״�������
����������ˮ�еļ״�������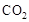 ��������ʵ��������ͼװ��ģ���������̣�
��������ʵ��������ͼװ��ģ���������̣�
��д�������缫��Ӧʽ ��
�ڳ�ȥ�״������ӷ�ӦΪ��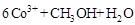
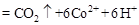 ���ù����б�������Ԫ���� ����������״����2.24L
���ù����б�������Ԫ���� ����������״����2.24L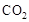 ʱ����ת�Ƶ��� mol��
ʱ����ת�Ƶ��� mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡΫ���и���3�µ�һ��ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ������
�״��� ������Ҫ����Դ���ʣ��о��״�������Ҫ���塣
������Ҫ����Դ���ʣ��о��״�������Ҫ���塣
��1�����ù�ҵ�����е� ����ȡ�״����䷴ӦΪ��
����ȡ�״����䷴ӦΪ��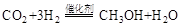
���³�ѹ����֪���з�Ӧ�������仯��ͼ��ʾ��

д���ɶ�����̼�������Ʊ��״����Ȼ�ѧ����ʽ��
��
��2��Ϊ��״�ȼ�ϵ������ʣ���ѧ�ҷ�����һ��ȼ�ϵ�أ���ص�һ���缫ͨ���������һ���缫ͨ��״����壬������Dz�����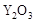 ��
�� 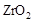 ���壬�ڸ��������ܴ���
���壬�ڸ��������ܴ���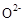 ���ӡ���ع���ʱ������ӦΪ ��
���ӡ���ع���ʱ������ӦΪ ��
���Ըõ��Ϊ��Դ����ʯī���缫���100mL�����������ӵ���Һ��
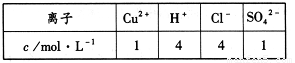
���һ��ʱ��������ռ�����ͬ�������ͬ������������ʱ��������Һ����ı仯���缫������ܴ��ڵ��ܽ������������ռ������������ʵ���Ϊ mol��
��3���״���ˮ�ʻ����һ������Ⱦ����һ�ֵ绯ѧ��������������Ⱦ����ԭ���ǣ�ͨ���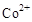 ������
������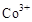 ��Ȼ����
��Ȼ����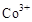 ����������ˮ�еļ״�������
����������ˮ�еļ״�������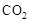 ��������ʵ��������ͼװ��ģ���������̣�
��������ʵ��������ͼװ��ģ���������̣�
��д�������缫��Ӧʽ ��
�ڳ�ȥ�״������ӷ�ӦΪ��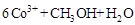
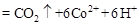 ���ù����б�������Ԫ���� ����������״����2.24L
���ù����б�������Ԫ���� ����������״����2.24L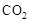 ʱ����ת�Ƶ��� mol��
ʱ����ת�Ƶ��� mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�갲��ʡʡ����У�������Ĵ�������ѧ�Ծ� ���ͣ�ѡ�������
��12�֣�Ϊ�������鶨�顷2012���һ��ŵ�ڵ��ں����������������⣬��������������˲�ͬ��Ŭ������������Դ�Ŀ��������ã�CH3OH��������������ǵ���Ұ��Խ��Խ�ܵ����ǵĹ�ע��
��1����ͼ����CO��g��+2H2��g����CH3OH��g�����й����е������仯���ߡ�����a��ʾ��ʹ�ô���ʱ��Ӧ�������仯������b��ʾ���������˵����ȷ���ǣ� ��
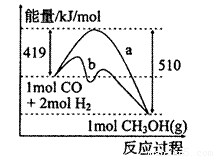
A���÷�Ӧ�����ȷ�Ӧ
B��ʹ�ô�����Ӧ�ȼ�С
C���Ȼ�ѧ����ʽΪCO��g��+2H2��g�� CH3OH��g�� ��H=-510kJ��mol
��2���ɣ�1���ƶϣ�CO��g��+2H2��g�� CH3OH(g)���ܱ������н��У�ͼ������a����һ�������¸÷�Ӧ�Ĺ��̡���ʹa���߱�Ϊb���ߣ��ɲ�ȡ�Ĵ�ʩ�ǣ� ��
CH3OH(g)���ܱ������н��У�ͼ������a����һ�������¸÷�Ӧ�Ĺ��̡���ʹa���߱�Ϊb���ߣ��ɲ�ȡ�Ĵ�ʩ�ǣ� ��
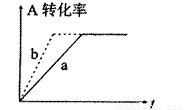
A������CO��Ũ�� B����С�������ݻ�
C��������� D�������¶�
��3���ɼ״��������Լ�ǿ�����������Һ������ȼ�ϵ�أ��������ƹ�ʹ�ã��ٶ��ŵ�����У��״���ȫ��������������̼�������������CO32-���õ�صĸ�����Ӧ�����ӷ���ʽΪ ���ŵ�����е������Һ��pH�� ����½����������������䡱��������16�˼״�����ȫ�����������ܣ������øù������ͷŵĵ��ܵ������������ͭ��Һ������������������Ϊ80������õ����������ʵ����� ��
��4��ijͬѧ���״���ȫȼ������CO2����ͨ��200mL 0��1 mol/L��ʯ��ˮ�õ�lg��������ôͨ���CO2���������Ϊ����̬�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com