
��16�֣�ÿ��2�֣���1����֪��ͪ[ CH3COCH3��]����ʽ�ɱ�ʾΪ ���ݼ���ʽ�ش��������⣺
���ݼ���ʽ�ش��������⣺ ����ʽ�� ���ṹ��ʽ��
����ʽ�� ���ṹ��ʽ��
��2�� ����������� ��ԭ�ӹ�ֱ�ߣ������ ��ԭ�ӹ�ƽ��
����������� ��ԭ�ӹ�ֱ�ߣ������ ��ԭ�ӹ�ƽ��
��3�����CH3CH(C2H5)CH(CH3)2��������
��4��д���ɱ���ϩ�� 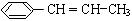 ���ڴ������������ɾ۱���ϩ�ķ�Ӧ����ʽ��
���ڴ������������ɾ۱���ϩ�ķ�Ӧ����ʽ��
��5�� �ж��ֵ�ͬ���칹�壬�������ڷ����������ͬ���칹�干��4�֣����ǵĽṹ��ʽ�ǣ� �� ������д���ж��֣�
�ж��ֵ�ͬ���칹�壬�������ڷ����������ͬ���칹�干��4�֣����ǵĽṹ��ʽ�ǣ� �� ������д���ж��֣�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��16�֣�ÿ��2�֣�ij�����Ĺ�ҵ��ˮ�к��д�����FeSO4���϶��CuSO4������Na2SO4��Ϊ�˼�����Ⱦ�����Ϊ���������ƻ��Ӹ÷�ˮ�л������������ͽ���ͭ���������������ͼ����ɻ�������������ͭ��ʵ�鷽�������ɹ�ѡ����Լ�Ϊ���ۡ�ϡH2SO4��NaOH��Һ���Լ�����1������a������Ϊ ������Ҫ�IJ�������Ϊ ____��
��2������E�ijɷ�Ϊ ____��������Լ���Ϊ __�������Ļ�ѧ����ʽΪ ��
��3�������Լ��ٵ�Ŀ���� ____________��
��4������ҺD����ҺG�еõ�FeSO4.7H2O����IJ���Ϊ ����ȴ�ᾧ ��
��ϴ�ӡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����������ݶ��и�һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��16�֣�ÿ��2�֣�ij�����Ĺ�ҵ��ˮ�к��д�����FeSO4���϶��CuSO4������Na2SO4��Ϊ�˼�����Ⱦ�����Ϊ���������ƻ��Ӹ÷�ˮ�л������������ͽ���ͭ������� ��������ͼ����ɻ�������������ͭ��ʵ�鷽�������ɹ�ѡ����Լ�Ϊ���ۡ�ϡH2SO4��
��������ͼ����ɻ�������������ͭ��ʵ�鷽�������ɹ�ѡ����Լ�Ϊ���ۡ�ϡH2SO4�� NaOH��Һ���Լ���
NaOH��Һ���Լ���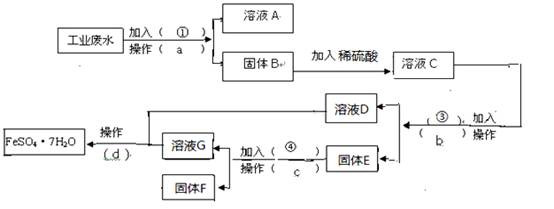 ��1������a������Ϊ ������Ҫ�IJ�������Ϊ ____��
��1������a������Ϊ ������Ҫ�IJ�������Ϊ ____��
��2������E�ijɷ�Ϊ  ____��������Լ���Ϊ __�������Ļ�ѧ����ʽΪ ��
____��������Լ���Ϊ __�������Ļ�ѧ����ʽΪ ��
��3�������Լ��ٵ�Ŀ ���� ____________��
���� ____________��
��4������ҺD����ҺG�еõ�FeSO4.7H2O����IJ���Ϊ ����ȴ�ᾧ ��
��ϴ�ӡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�������и�һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��16�֣�ÿ��2�֣�ij�����Ĺ�ҵ��ˮ�к��д�����FeSO4���϶��CuSO4������Na2SO4��Ϊ�˼�����Ⱦ�����Ϊ���������ƻ��Ӹ÷�ˮ�л������������ͽ���ͭ���������������ͼ����ɻ�������������ͭ��ʵ�鷽�������ɹ�ѡ����Լ�Ϊ���ۡ�ϡH2SO4��NaOH��Һ���Լ��� ��1������a������Ϊ
������Ҫ�IJ�������Ϊ
____��
��1������a������Ϊ
������Ҫ�IJ�������Ϊ
____��
��2������E�ijɷ�Ϊ ____��������Լ���Ϊ __�������Ļ�ѧ����ʽΪ ��
��3�������Լ��ٵ�Ŀ���� ____________��
��4������ҺD����ҺG�еõ�FeSO4.7H2O����IJ���Ϊ ����ȴ�ᾧ ��
��ϴ�ӡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�켪��ʡ���������ѧУ�߶���ѧ�����п��Ի�ѧ�������Ծ� ���ͣ�ʵ����
��16�֣�ÿ��2�֣�����������Ϊ98%���ܶ�Ϊ1.84 g•cm-3��Ũ��������100mL���ʵ���Ũ��Ϊ1mol/L��ϡ���ᡣ
��1����Ҫʹ�õ���Ҫ��������Ͳ���ձ����������� �� ��
��2�����������ɷֽ�Ϊ���¼�����
A������Ͳ��ȡ mLŨ���ᣬ����ע��װ��Լ50mL����ˮ���ձ�����ò���������
B����Լ30mL����ˮ������ϴ���ձ��Ͳ���������ÿ�ε�ϴҺ����������ƿ��
C����ϡ�ͺ������С�ĵ��ò�������������ƿ��
D�����100mL����ƿ���Ƿ�©ˮ
E��������ˮֱ�Ӽ�������ƿ����Һ��ӽ��̶���
F���ǽ�ƿ���������ߵ���ҡ����Һ
G���ý�ͷ�ι�������ƿ����μ�������ˮ����Һ����͵�ǡ�����������
��ݴ���д��
��������������еĿհ״���
����ȷ�IJ���˳���ǣ�����ĸ��д����
�� ������ ������ ������ ������ ������ ������ ����
�۽���A������ʱ��ѡ����Ͳ�Ĺ���� ����ѡ����ĸ��
A��10mL B��50mL C��100mL D��1000mL
�������װ��Ũ�������Ͳ���Ӷ��������Ƶ�ϡ����Ũ�Ƚ� ��ѡ�ƫ�ߡ�����ƫ�͡�������Ӱ�족����
��A����������ʱ������ ���ܽ��к���IJ�����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com