
��10�֣���λ��ѧ�ǻ�ѧ��һ����Ҫ��֧����ʮ�����ͣ�ά���ɵ���ʦ��Ϊ������λ��������һ����ʽ�ṹ���磺M�DA�DB�DC�DD�DE�DF��M�DB�DA�DC�DD�DE???? ����ά������Ϊ������λ��������һ�ְ����塣
4-1 ��������һ�£�ά��������η���ʦ���۶ϵó�������ȷ���۵ģ�
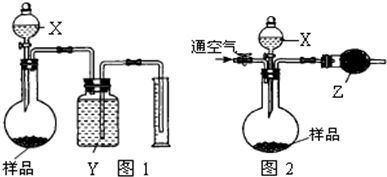
��������У�������ͪ��CH3COCH2COCH3���dz�����һ�����塣������ش��������⣺
4-2 ������ͪ��![]() ������˫�����塣����л���ѧ�ĽǶȼ��Խ��͡�
������˫�����塣����л���ѧ�ĽǶȼ��Խ��͡�
4-3 ijͬѧ��������ͪ��CoCl2��H2O2��һ�������·�Ӧ�õ�һ����A��Ϊ�ⶨ������ɣ���ͬѧ�������ʵ�飺ȷ��ȡA����0.4343g������ܽ���������ӽ�����֬����HR�ͣ���ֽ�������������Co2���ĺ�ɫ�����ˣ���0.1013mol/L��NaOH����Һ�ζ�����ȥ24.07mL����ԭ�������ã�Co��58.93��H��1.01��C��12.01��O16.00��CI��35.45����
4-1 A��Co������������
4-2 Ԥ��A�Ļ�ѧʽ������A�Ľṹ��д��Co���ӻ���̬��
4-3 д���Ʊ�A�Ļ�ѧ��Ӧ����ʽ��
4-4 �����۵Ĵ��£�A����Һ�巢��ȡ����Ӧ����д���仯ѧ��Ӧ����ʽ��������ԭ��
4-1 ��ʽ�ṹ�������칹�壬����������������칹��ϵ��������ij������������Dz���������ԣ�����λ��Ϊ6����������һ���ǰ�����ṹ����2�֣�
4-2 ������֮ͪ���Կ���Ϊ˫�����壬����Ϊ������ʼ�մ���ϩ��ʽ��ͪʽ�Ļ����칹��
![]()
![]()
![]() ��1�֣�
��1�֣�
4-3 ��1��n (Co)��![]() n (H��)��
n (H��)��![]() ��
��![]() ��
��![]() ��0.001219mol��
��0.001219mol��
MA��![]() ��Co%��
��Co%��![]() ��2�֣�
��2�֣�
��2����MA�Ƴ�ӦΪCo(C5H7O2)3��ע�ⲻ��дΪCo(C5H8O2)3��Co��sp3d 2��
 ��2�֣�
��2�֣�
��3��CH3COCH2COCH3+CoCl2+2H2O2��Co(CH3COCHCOCH3)3+H2O+2HCl+1/2O2��1�֣�
��4�� +3Br2
+3Br2![]()
 + 3HBr��������������ԭ���������ʻ��������£����ֳ��������ԣ������������������±�Br�� ȡ������2�֣�
+ 3HBr��������������ԭ���������ʻ��������£����ֳ��������ԣ������������������±�Br�� ȡ������2�֣�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| m |
| ||
| m |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���㽭ʡ������һ���¿���ѧ�Ծ��������棩 ���ͣ������
��10�֣������������ʵ���Ҫ���������ʵĽṹ����ش��������⣺
��1����֪A��BΪ��������Ԫ�أ���ԭ�ӵĵ�һ�����ĵ��������±���ʾ��
|
������/kJ��mol��1 |
I1 |
I2 |
I3 |
I4 |
|
A |
578 |
1817 |
2745 |
11578 |
|
B |
738 |
1451 |
7733 |
10540 |
Aͨ���� �� �ۣ�A�ĵ縺�� �� B�ĵ縺��(�����������������)��
��2����֪������Ϊ300 nm�������Ĺ��ӣ����ӵ���������Ƶ�ʵĹ�ϵΪE��hv��ʽ��h��6.63��10��34J��s����IJ��� ����Ƶ��v�Ĺ�ϵΪ
����Ƶ��v�Ĺ�ϵΪ �����й���c=3��108m��s-1�������±��йص����ʷ�������Ҫ��ѧ������Ϣ����Ϊ300 nm�������Ĺ��������е��������������� kJ��mol��1��˵�����峤ʱ������������Ƥ���Ƿ�����˺���ԭ�� ������������������δ˵��ԭ���֣�
�����й���c=3��108m��s-1�������±��йص����ʷ�������Ҫ��ѧ������Ϣ����Ϊ300 nm�������Ĺ��������е��������������� kJ��mol��1��˵�����峤ʱ������������Ƥ���Ƿ�����˺���ԭ�� ������������������δ˵��ԭ���֣�
|
���ۼ� |
C��C |
C��N |
C��S |
|
����/kJ��mol��1 |
347 |
305 |
259 |
��3����ѧ��ͨ��X����̽����KCl��MgO��CaO��TiN�ľ���ṹ��NaCl�ľ���ṹ���ơ��±���3�����Ӿ���ľ��������ݣ�
|
���Ӿ��� |
NaCl |
KCl |
CaO |
|
������/kJ��mol��1 |
786 |
715 |
3401 |
���Ӽ���ǿ�����������Ӿ���ľ�������������KCl��CaO��TiN 3�����Ӿ����۵�Ӹߵ��͵�˳���� �� ��MgO������һ��Mg2����Χ�������ڽ��ҵȾ����Mg2���� �� ����
��4���о����ʴ��Ա��������������Ӻ�δ�ɶԵ���Խ�࣬�����Խ�ż�¼����Խ�á�������������V2O5��CrO2�У��ʺ���¼�����ŷ�ԭ�ϵ��� �� ��
��5��ij�����ķ��ӽṹ��ͼ��ʾ��������ڲ����� (����ĸ)��

A�����Ӽ� B�����ۼ� C����λ�� D�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���㽭ʡ������һ��ͳ����ѧ�Ծ��������棩 ���ͣ������
��8�֣������������ʵ���Ҫ���������ʵĽṹ����ش��������⣺
��1����֪A��BΪ��������Ԫ�أ���ԭ�ӵĵ�һ�����ĵ��������±���ʾ��
|
������/kJ��mol��1 |
I1 |
I2 |
I3 |
I4 |
|
A |
578 |
1817 |
2745 |
11578 |
|
B |
738 |
1451 |
7733 |
10540 |
Aͨ���� �� �ۣ�A�ĵ縺�� �� B�ĵ縺��(�����������������)��
��2����֪������Ϊ300 nm�������Ĺ��ӣ����ӵ���������Ƶ�ʵĹ�ϵΪE��hv��ʽ��h��6.63��10��34J��s����IJ��� ����Ƶ��v�Ĺ�ϵΪ
����Ƶ��v�Ĺ�ϵΪ �����й���c=3��108m��s-1�������±��йص����ʷ�������Ҫ��ѧ������Ϣ����Ϊ300 nm�������Ĺ��������е��������������� kJ��mol��1��˵�����峤ʱ������������Ƥ���Ƿ�����˺���ԭ��
�����й���c=3��108m��s-1�������±��йص����ʷ�������Ҫ��ѧ������Ϣ����Ϊ300 nm�������Ĺ��������е��������������� kJ��mol��1��˵�����峤ʱ������������Ƥ���Ƿ�����˺���ԭ��
���������������������������� ������������������ ����δ˵��ԭ���֣�
|
���ۼ� |
C��C |
C��N |
C��S |
|
����/kJ��mol��1 |
347 |
305 |
259 |
��3����ѧ��ͨ��X����̽����KCl��MgO��CaO��TiN�ľ���ṹ��NaCl�ľ���ṹ���ơ��±���3�����Ӿ���ľ��������ݣ�
|
���Ӿ��� |
NaCl |
KCl |
CaO |
|
������/kJ��mol��1 |
786 |
715 |
3401 |
���Ӽ���ǿ�����������Ӿ���ľ�������������KCl��CaO��TiN 3�����Ӿ����۵�Ӹߵ��͵�˳���� �� ��MgO������һ��Mg2����Χ�������ڽ��ҵȾ����Mg2���� �� ����
��4���о����ʴ��Ա��������������Ӻ�δ�ɶԵ���Խ�࣬�����Խ�ż�¼����Խ�á�������������V2O5��CrO2�У��ʺ���¼�����ŷ�ԭ�ϵ��� �� ��
��5��ij�����ķ��ӽṹ��ͼ��ʾ��������ڲ����� (����ĸ)��

A�����Ӽ� B�����ۼ� C����λ�� D�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����λ��ѧ��ʼ��ά���ɷ��֣��������ʵ�����CoCl3?6NH3����ɫ����CoCl3?5NH3���Ϻ�ɫ����CoCl3?4NH3����ɫ����CoCl3?4NH3����ɫ�����ֻ���������ˮ������������������Һ�����������Ȼ������������ֱ�Ϊ3mol��2mol��1mol��1mol��
�������ʵ����ʵ����������ʽд�����ǵĻ�ѧʽ��
CoCl3?6NH3 CoCl3?5NH3
CoCl3?4NH3����ɫ����ɫ����
�ں��������������ͬ����ɫ��ͬ��ԭ����
������������У��������ӵ���λ������
�����ɫ�����Ȼ�����Һ�м�����ɫ��KSCN��Һ����Һ���Ѫ��ɫ���÷�Ӧ���еĽ̲����÷���ʽFeCl3+3KSCN=Fe��SCN��3+3KCl��ʾ�����о�������Fe��KSCN��3������Fe3+��SCN�D��������1��3�ĸ�������ϣ�������������������ϡ��밴Ҫ����գ�
��Fe3+��SCN�D��Ӧʱ��Fe3+�ṩ ��SCN�D�ṩ ������ͨ����λ����ϡ�
������Fe3+��SCN�D��������У���Ҫ��Fe3+��SCN�D�Ը�����1��1�������������Ѫ��ɫ���������ӵ������Ļ�ѧʽ�� ��
���� Fe3+��SCN�D�Ը�����1��5��ϣ���FeCl3��KSCN��ˮ��Һ�з�����Ӧ�Ļ�ѧ����ʽ���Ա�ʾΪ ��
����֪SCN�D����������ԭ�Ӿ����������8���ӽṹ��һ����������Sԭ�����ã���SCN�D�ĵ���ʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com