
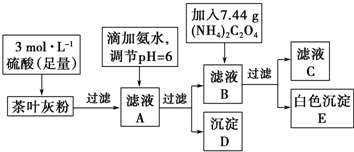
���� �ⶨij��Ҷ�и�Ԫ�ص�����������������Ԫ�صĴ��ڣ�ȡ200g��Ҷ��Ʒ���յûҷۣ���Ҷ�ҷ��м���3mol/L������������Һ�����˵õ���ҺA����Ҫ�Ǹ����ӡ������ӵȣ����백ˮ������ҺPH=6��ʹ������ȫ������������������D�����˵õ���Һ�м�������7.44g�����˵õ���ɫ����EΪ����ƣ���ҺCΪ�������Һ��
��1��Ϊ��ҷ��иơ��������ʿ��Խ��衢���¡��ӳ���ȡʱ��ȴ�ʩ���ӿ�������ʣ�
��2��pH��ֽ������ҺpH�ķ�����ȡһС��pH��ֽ�ڲ���Ƭ�ϣ��ò�����պȡ��Һ������pH��ֽ���룬���ݱ�ɫ�ȶ���ҺPH��
��3��������ҺA������D�������ӺͰ�ˮ��Һ��һˮ�ϰ���Ӧ����һˮ�ϰ�����Σ�
��4��ϴ�ӳ���D��Ҫ�������һ��ϴ��Һ���Ƿ���������������ʵ����֤��
��5������DΪ��������������ϡ�����ܽ�����KSCN��Һ�����Ƿ��������Ӵ��ڣ�
��6������Ksp��CaC2O4��=c��C2O42-����c��Ca2+�����㣮һ���¶����ܶȻ��dz�����
��� �⣺�ⶨij��Ҷ�и�Ԫ�ص�����������������Ԫ�صĴ��ڣ�ȡ200g��Ҷ��Ʒ���յûҷۣ���Ҷ�ҷ��м���3mol/L������������Һ�����˵õ���ҺA����Ҫ�Ǹ����ӡ������ӵȣ����백ˮ������ҺPH=6��ʹ������ȫ������������������D�����˵õ���Һ�м�������7.44g�����˵õ���ɫ����EΪ����ƣ���ҺCΪ�������Һ��
��1��Ϊ��ҷ��иơ��������ʿ��Խ��衢���¡��е�����Ũ�ȡ��ӳ���ȡʱ��ȴ�ʩ���ӿ�������ʣ�
�ʴ�Ϊ�����裨�ʵ����ȣ��ʵ��������Ũ�ȣ��ӳ���ȡʱ��ȣ���
��2��pH��ֽ������ҺpH�ķ�����ȡһС��pH��ֽ�ڲ���Ƭ�ϣ��ò�����պȡ��Һ������pH��ֽ���룬���ݱ�ɫ�ȶ���ҺPH�����岽��Ϊ��ȡһС��pH��ֽ�ڲ���Ƭ�ϣ��ò�����պȡ��Һ������pH��ֽ���룬����ɫ�������ɫ���Աȶ�����
�ʴ�Ϊ��ȡһС��pH��ֽ�ڲ���Ƭ�ϣ��ò�����պȡ��Һ������pH��ֽ���룬����ɫ�������ɫ���Աȶ�����
��3��������ҺA������D�������ӺͰ�ˮ��Һ��һˮ�ϰ���Ӧ����һˮ�ϰ�����Σ���Ӧ�����ӷ���ʽΪ��Fe3++3NH3•H2O�TFe��OH��3��+3NH4+��
�ʴ�Ϊ��Fe3++3NH3•H2O�TFe��OH��3��+3NH4+��
��4��ϴ�ӳ���D��Ҫ�������һ��ϴ��Һ���Ƿ��������������ƣ�ʵ����֤鱗���Ϊ��ȡ���һ��ϴ�ӹ���Һ�������Թ��У��μ�BaCl2��Һ�����ް�ɫ��������������ϴ�Ӹɾ���
�ʴ�Ϊ��ȡ���һ��ϴ�ӹ���Һ�������Թ��У��μ�BaCl2��Һ�����ް�ɫ��������������ϴ�Ӹɾ���
��5������DΪ��������������ϡ�����ܽ�����KSCN��Һ�����Ƿ��������Ӵ��ڣ�ʵ�����Ϊ��ȡ��������D���Թ��У���ϡ�����ܽ⣬����Һ�м����軯�أ�����Һ��Ѫ��ɫ��������Ԫ�أ�
�ʴ�Ϊ��ȡ��������D���Թ��У���ϡ�����ܽ⣬����Һ�м����軯�أ�����Һ��Ѫ��ɫ��������Ԫ�أ�
��6��CaCl2��Һ��Ũ��Ϊ2��10-4mol/L���������Ϻ�Ũ��Ϊ1��10-4mol/L��
��Ksp��CaC2O4��=c��C2O42-����c��Ca2+����֪����Ϻ�c��C2O42-��=$\frac{Ksp}{c��C{a}^{2+}��}$=$\frac{2.32��1{0}^{-9}}{1��1{0}^{-4}}$=2.32��10-5mol•L-1
��������Na2C2O4��Һ����С��ʼŨ��Ϊ2.32��10-5mol•L-1��2=4.64��10-5 mol•L-1��
�ʴ�Ϊ��4.64��10-5 mol•L-1��
���� ���⿼����̽����Ҷ����Ԫ�ء���Ԫ�صĺ����ķ���������ʵ�鷽������ƣ���Ŀ�Ѷ��еȣ�ע����������ʵ�鷽������Ʒ�������ȷ̽��������ɼ������ķ���������������ѧ�����Ӧ����ѧ֪ʶ���ʵ�������������


 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
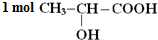 �ֱ���Na��NaOH��NaHCO3��ȫ��Ӧʱ�����ĵ�Na��NaOH��NaHCO3�����ʵ���֮��Ϊ2��1��1��д�����л����NaOH��Ӧ�Ļ�ѧ����ʽCH3CH��OH��COOH+NaOH��CH3CH��OH��COONa+H2O��
�ֱ���Na��NaOH��NaHCO3��ȫ��Ӧʱ�����ĵ�Na��NaOH��NaHCO3�����ʵ���֮��Ϊ2��1��1��д�����л����NaOH��Ӧ�Ļ�ѧ����ʽCH3CH��OH��COOH+NaOH��CH3CH��OH��COONa+H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���

 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �ڢ� | C�� | �ڢ� | D�� | �ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڢܢ� | B�� | �٢ۢ� | C�� | �٢� | D�� | �٢ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
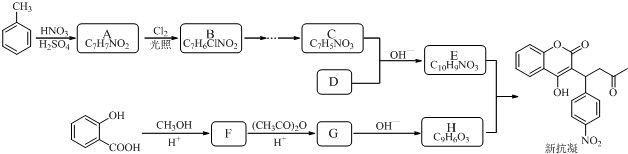
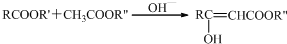
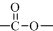 �ṹ��
�ṹ��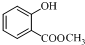 +��CH3CO��2O$\stackrel{H+}{��}$
+��CH3CO��2O$\stackrel{H+}{��}$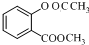 +CH3COOH��
+CH3COOH�� ��
�� $\stackrel{OH-}{��}$
$\stackrel{OH-}{��}$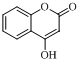 +CH3OH��
+CH3OH���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
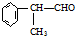 �����ʣ���������һ�����ϣ�
�����ʣ���������һ�����ϣ�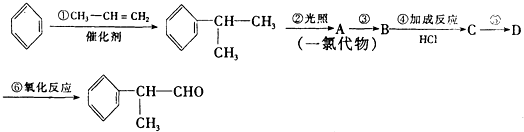
 ��
�� +O2$��_{��}^{����}$2
+O2$��_{��}^{����}$2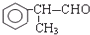 +2H2O��
+2H2O��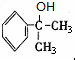 ����
���� ��
�� ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SO2�ɴ�������Ư��ʳƷ | B�� | �ɱ���AgI���������˹����� | ||
| C�� | ������������������ɫ�����Ϳ�� | D�� | С�մ����Ҫ�ɷ���Na2CO3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com