
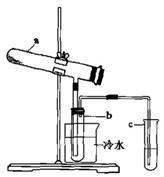
 Fe2O3+SO2+SO3��+14H2O����������������ˮ����ͬʱ������õ����ᡣ����ͼ��ʾװ��ģ�����̷��������ʵ�飬���������ɵ�����Ͷ���������װ������ȥ��������bΪ������Թ�
Fe2O3+SO2+SO3��+14H2O����������������ˮ����ͬʱ������õ����ᡣ����ͼ��ʾװ��ģ�����̷��������ʵ�飬���������ɵ�����Ͷ���������װ������ȥ��������bΪ������Թ� ��
��
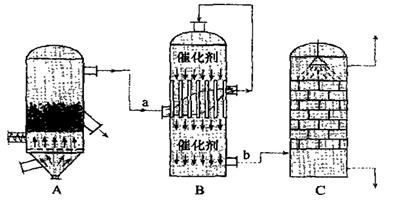
| A����������ĽӴ������ںϳ����з��� |
| B���������õ�������Ũ��Ϊ98�� |
| C�����պ���48���Ļ�����ʱ����FeS2��ʧ��2������S��ʧ4�� |
D�� Bװ���з�Ӧ������֮һΪ�ϸ��¶���Ϊ�����SO2��ת���� Bװ���з�Ӧ������֮һΪ�ϸ��¶���Ϊ�����SO2��ת���� |
 ���� ���� ���ɲ���������
���� ���� ���ɲ���������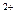 +SO
+SO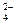 =BaSO4����1�֣�
=BaSO4����1�֣�

 �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
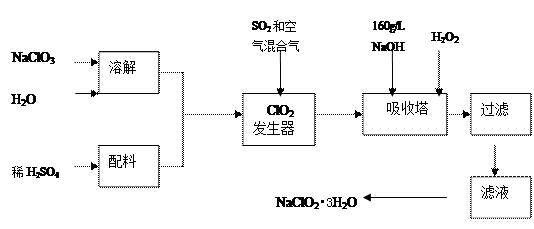

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
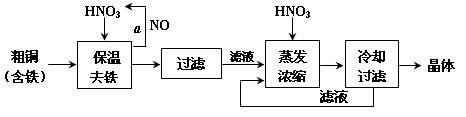
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2NH3��g������
2NH3��g������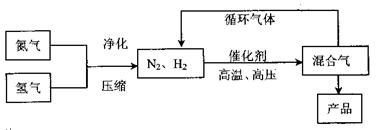
 2NH3��g���ġ�H= ��
2NH3��g���ġ�H= ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

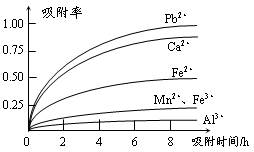
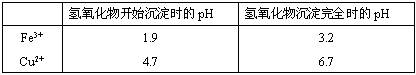
| ���� | ���Ӱ뾶(pm) | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe2�� | 74 | 7.6 | 9.7 |
| Fe3�� | 64 | 2.7 | 3.7 |
| Al3�� | 50 | 3.8 | 4.7 |
| Mn2�� | 80 | 8.3 | 9.8 |
| Pb2�� | 121 | 8.0 | 8.8 |
| Ca2�� | 99 | �� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
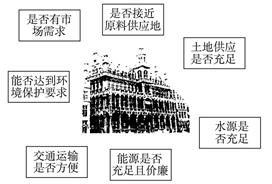
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
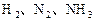 ��Ӧ��ƽ��ʱ������Ũ������һ���ǣ� ��
��Ӧ��ƽ��ʱ������Ũ������һ���ǣ� ��| | 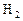 | 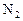 | 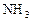 |
| A | 6 | 2 | 0 |
| B | 1 | 0 | 4 |
| C | 3.5 | 1 | 2 |
| D | 5 | 1.5 | 1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��(1)(3)(5) | B��(2)(3)(4) | C��(1)(3)(4) | D��(1)(3)(4)(5) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ϳɰ������н�NH3Һ�����룬�ɼӿ�����Ӧ���ʣ����H2��ת���� |
| B�����Ṥҵ�У��Ӵ������Ƚ�����������SO3ת��ΪH2SO4ʱ�ų������� |
| C���ȼҵ�������ӽ���Ĥ�����ɷ�ֹ�����Ҳ�����Cl2���������� |
| D����������������ʴʱ�������ķ�ӦʽΪ��Fe-2e��Fe2+ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com