
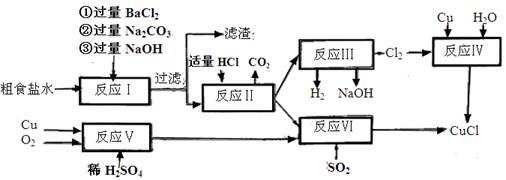
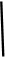 ӣ
”£ 2NaOH£«H2”ü£«Cl2”ü
2NaOH£«H2”ü£«Cl2”ü

 ½Ģ²ÄČ«½ā×Ö“Ź¾äĘŖĻµĮŠ“š°ø
½Ģ²ÄČ«½ā×Ö“Ź¾äĘŖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®5”«9 | B£®4.0”«10.0 | C£®4.3”«9.7 | D£®ŅŌÉĻ¶¼²»ÕżČ· |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
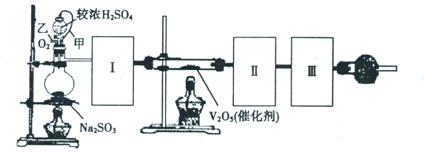
| ±ąŗÅ | ŹµŃéÄŚČŻ | ŹµŃéŌĄķ | ·¢Éś×°ÖĆ |
| ¢Ł | ÖĘŃõĘų |  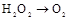 | |
| ¢Ś | ÖĘ°±Ęų | 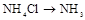 | |
| ¢Ū | ÖĘĀČĘų | 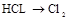 | |
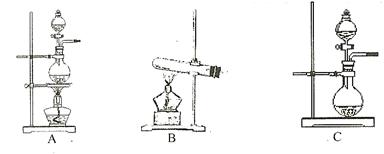
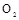 µÄ×°ÖĆÖʱø
µÄ×°ÖĆÖʱø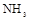 ,ӦєŌńµÄŹŌ¼ĮĪŖ .
,ӦєŌńµÄŹŌ¼ĮĪŖ .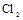 ŠčÓĆ
ŠčÓĆ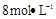 µÄŃĪĖį1OOml,ĻÖÓĆ
µÄŃĪĖį1OOml,ĻÖÓĆ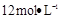 µÄŃĪĖįĄ“ÅäÖĘ”£
µÄŃĪĖįĄ“ÅäÖĘ”£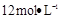 µÄŃĪĖįµÄĢå»żĪŖ mL£»
µÄŃĪĖįµÄĢå»żĪŖ mL£»²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 (NH4)2S2O8+H2”ü£¬
(NH4)2S2O8+H2”ü£¬ 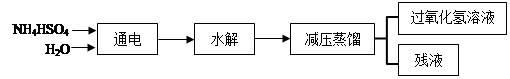
| A£®²»ĪČ¶ØŠŌ | B£®Čõ¼īŠŌ | C£®Ńõ»ÆŠŌ | D£®»¹ŌŠŌ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
| A£®Īü³ö¶ąÓąŅŗĢ壬Ź¹°¼ŅŗĆęÓėæĢ¶ČĻßĻąĒŠ |
| B£®Š”ŠÄ¼ÓČČČŻĮæĘ棬¾Õō·¢ŗó£¬Ź¹°¼ŅŗĆęÓėæĢ¶ČĻßĻąĒŠ |
| C£®¾¼ĘĖć¼ÓČėŅ»¶ØĮæµÄÅØĮņĖį |
| D£®ÖŲŠĀÅäÖĘ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
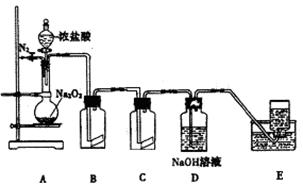
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com