���ڹ�����ռ����Ҫ��λ����ش��йذ����������⣺
��1����֪��N
2��g��+O
2��g���T2NO��g����H=+akJ/mol
4NH
3��g��+5O
2��g���T4NO��g��+6H
2O��g����H=-bkJ/mol
2H
2��g��+O
2��g���T2H
20��g����H=-ckJ/mol
��N
2��g��+3H
2��g��?2NH
3��g���ġ�H=
��
��2����һ���¶��£���3molN
2�����7molH
2����ͨ�뵽���Ϊ1L���ܱ������У�����Ӧ�ﵽƽ��ʱ�������������ѹǿΪ��ʼʱ��80%������ƽ�ⳣ��Ϊ
���ı�������������ʹƽ��������Ӧ���������ƽ�ⳣ���������
�٢�
�٢�
��
������ѹǿ ������Ӧ���ʵ�Ũ�� ��ʹ�ô��� �ܽ����¶�
��3���������������������Ͱ����Ĺܵ��Ƿ�©�������©������а��̣��ɷ�Ϊ�Ȼ�泥����ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ
8NH3+3Cl2=N2+6NH4Cl
8NH3+3Cl2=N2+6NH4Cl
��
��4����ҵ������ˮ�����ʵ���Ũ��Ϊ20mol/L��ʵ����������80mLŨ��Ϊ5mol/L�İ�ˮʱ����ȡ20mol/L�İ�ˮ
25mL
25mL
mL����100mL������ƿ��������ð�ˮ��pH=a��������ͬ���������ʱ������Һǡ�÷�Ӧ����������pH
��
��
14-a������ڡ���С�ڡ����ڡ�����





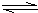 2NH3�ġ�H
2NH3�ġ�H