
����Ŀ��ij��ҵ��ˮ�����±��е�ijЩ���ӣ��Ҹ������ӵ����ʵ���Ũ����ȣ���Ϊ0.1 mol/L(����ֵ����ˮ�ĵ��뼰���ӵ�ˮ��)��
������ | K����Ag����Mg2����Cu2����Al3����NH4+ |
������ | Cl����CO32����NO3����SO42����SiO32����I�� |
��ͬѧ��̽����ˮ����ɣ�����������ʵ�飺
��.ȡ����ɫ��Һ5 mL���μ�һ�ΰ�ˮ�г������ɣ��������������ӡ�
��.�ò�˿պȡ��Һ���ڻ��������գ�����ɫ�ܲ����۲죬����ɫ���档
��.��ȡ��Һ����������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ��
��.��������õ���Һ�м���BaCl2��Һ���а�ɫ�������ɡ�
���ƶϣ�
(1)�ɢ��жϣ���Һ��һ�������е���������____________��
(2)���м�������������ɫ��������ӷ���ʽ��_____________________________��
(3)��ͬѧ����ȷ��ԭ��Һ��������������________����������________�����ݴ��Ʋ�ԭ��ҺӦ�ó�_______________________________________________�ԣ�ԭ����_________________________________(�������ӷ���ʽ˵��)��
(4)��ȡ100 mLԭ��Һ������������NaOH��Һ���˹������漰�����ӷ���ʽΪ__________________________________________________________����ַ�Ӧ����ˣ�ϴ�ӣ����ճ��������أ��õ��Ĺ�������Ϊ________g��
���𰸡�K����NH4+��Cu2�� 6I����2NO3����8H����3I2��2NO����4H2O Mg2����Al3�� Cl����NO3����SO42����I�� �� Mg2����2H2O![]() Mg(OH)2��2H����Al3����3H2O
Mg(OH)2��2H����Al3����3H2O![]() Al(OH)3��3H�� Mg2����2OH��=Mg(OH)2����Al3����4OH��=AlO2����2H2O 0.4
Al(OH)3��3H�� Mg2����2OH��=Mg(OH)2����Al3����4OH��=AlO2����2H2O 0.4
��������
(1)���ݢ���ҺΪ��ɫ������Һ�в���Cu2�������ɳ����������������ӣ�˵��ԭ��Һ�в���NH4+�����ݢ��ȷ����Һ�в���K����
(2)���ݢ��������ȷ�����ɵ���ɫ����ΪNO����ԭ��Һ��һ������NO3����I��������ת�Ƶ�����Ŀ��ȡ�����غ��д�����ӷ���ʽΪ6I����2NO3����8H����3I2��2NO����4H2O��
(3)��Ϣ�����Һ�и�����Ũ����ȿ�ȷ����Һ�к��е�������ΪSO42����Cl����NO3����I������Һ�к��е�������ΪMg2����Al3���������Һ����ɿ�ȷ����Һ�����ԣ���Mg2����Al3��ˮ���Ե�ʣ�����ʽΪMg2����2H2O![]() Mg(OH)2��2H����Al3����3H2O
Mg(OH)2��2H����Al3����3H2O![]() Al(OH)3��3H����
Al(OH)3��3H����
(4)����NaOH��Һ��Mg2��ת��ΪMg(OH)2������Al3�����ת��ΪNaAlO2������ʽΪMg2����2OH��=Mg(OH)2����Al3����4OH��=AlO2����2H2O�����յõ�0.01 mol Mg(OH)2���������������صõ�0.01molMgO��������0.4 g��


 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д� �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ����٤��������ֵ������˵����ȷ����
A.��״���£�0.1mol Cl2����ˮ��ת�Ƶĵ�����ĿΪ0.1NA
B.��״���£�2.24L NO��2.24L O2��Ϻ����������Ϊ0.15 NA
C.0.1mol Na2O2�������ij�ʪ�Ķ�����̼��Ӧת�Ƶĵ�����Ϊ0.1NA
D.���������£�1mol FeͶ��������Ũ�����У�����NA��SO2����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����16gO2����ԭ��������ͬ��NH3��״���������________L��
��2����֪2L Al2(SO4)3��Һ��c(Al3+)=3mol/L������3L__________mol/LNa2SO4��SO42�������ʵ���Ũ����ȡ�
��3��ͬ��ͬѹ�£�ͬ�����NH3��H2S�����������Ϊ_____________��ͬ������NH3��H2S����������Ϊ___________�����к��е����ԭ�Ӹ�����Ϊ________����NH3��H2S�е���ԭ������ȣ����ǵ������Ϊ____________��
��4���ڱ�״���£�8.96L��CH4��CO�Ļ�����壬����������������ܶ���9.5������������CH4�����Ϊ____________��CH4��COԭ�Ӹ�����Ϊ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ɸ�������ɺ����ʽ��з��ࣺ
��1��������ʾ�����ʷ���������� ______ ��
��2����CO2 ��Cu ��FeCl3��Һ ��H2SO4 �����������彺�� ��Al2(SO4)3���� ���ƾ� ��BaSO4����
���ڵ���ʵ��� ______ �����ڷǵ���ʵ��� ______ ������ţ���
��3����д�����ĵ��뷽��ʽ______________________________________________
��4�����й�����������˵����ȷ����____________��
a�������ȶ����ܷ⾲�û��������
b�����ܲ��������ЧӦ����������
c���������ᶼ���������
��5����Ҫ��д�����з�Ӧ�����ӷ���ʽ��
��п��ϡ���ᷴӦ_________________________________________________��
������������Һ��ϡ���ᷴӦ ______________________________________��
��MgO�μ�ϡ����_______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������̫���ֽܷ�ˮ���⣬�����0.02 molˮ������˵����ȷ����
A.������H2������Ϊ0.02g
B.���������ԭ����Ϊ2.408��1023��
C.������H2�����Ϊ0.224 L(�����)
D.����H2���������ϵ���0.48 g Mg������ϡ���ᷴӦ����H2����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ��д��ȷ����(����)
A. FeCl3��Һ��ʴӡˢ��·ͭ�壺Cu��Fe3��=Cu2����Fe2��
B. �����ܽ⼦���ǣ�2H����CaCO3=Ca2����CO2����H2O
C. ��NaHSO4��Һ�е���Ba(OH)2��Һ����Һ�����ԣ�Ba2����2OH����2H����SO![]() =BaSO4����2H2O
=BaSO4����2H2O
D. ��NaHCO3��Һ�е�����������ʯ��ˮ��HCO![]() ��Ca2����OH��=CaCO3����H2O
��Ca2����OH��=CaCO3����H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ɼ������ӻ�������ɵĻ�����к������������е������֣�K����Cl����NH![]() ��Mg2����Ba2����CO
��Mg2����Ba2����CO![]() ��SO
��SO![]() �����û��������ˮ��ó�����Һ����ȡ����100 mL����Һ�ֱ��������ʵ�顣
�����û��������ˮ��ó�����Һ����ȡ����100 mL����Һ�ֱ��������ʵ�顣
ʵ����� | ʵ������ | ʵ���� |
1 | ����AgNO3��Һ | �а�ɫ�������� |
2 | ��������NaOH��Һ������ | �ռ�������1.12 L(��״����) |
3 | ��������BaCl2��Һ�������ó�������ϴ�ӡ������������������м�������ϡ���ᣬȻ����ˡ�ϴ�ӡ�������� | ��һ�γ�������Ϊ6.27 g���ڶ��γ�������Ϊ2.33 g |
��ش��������⣺
��1������ʵ��1��Cl���Ƿ���ڵ��ж���________(�һ�����ڡ���һ�������ڡ�����ȷ����)������ʵ��1��3�ж�ԭ�������һ�������ڵ�������________��
��2����ȷ��100 mL��Һ��һ�����ڵ������Ӽ������ʵ���Ũ��(�ɲ�����)��
�����ӷ��� | ���ʵ���Ũ��(mol��L��1) |
_______ | ___________ |
______ | _______________ |
��3��K���Ƿ���ڣ�________(����ڡ������ڡ�)���жϵ�������____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��ѧ��Ӧ�����е������仯����ͼ�ж�����˵���к������ǣ�������

A. 500 mL 2.0 mol��L��1HCl��Һ��500 mL 2.0 mol��L��1NaOH��Һ�ķ�Ӧ����ͼ��a�����ҷų�����Ϊ��E1
B. 500 mL 2.0 mol��L��1H2SO4��Һ��500 mL 2.0 mol��L��1Ba��OH��2��Һ�ķ�Ӧ����ͼ��b��������������Ϊ��E2
C. ����ͼ��a�������仯���κη�Ӧ��һ������Ҫ���ȼ��ɷ���
D. CaO��Ũ����ֱ�����ˮʱ�������仯������ͼ��a��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪����P4O6�ķ��ӽṹ��ͼ��ʾ����֪��ѧ���ļ������γ�(��Ͽ�)1 mol��ѧ��ʱ�ͷ�(������)���������ֲ�֪P��P����Ϊ198 kJ��mol��1��P��O����Ϊ360 kJ��mol��1��O===O����Ϊ498 kJ��mol��1��������1 mol P4O6����ӦP4(����)��3O2=P4O6�е������仯Ϊ( )
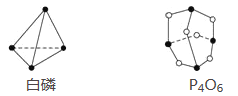
A. ����1 638 kJ���� B. �ų�1 638 kJ����
C. ����126 kJ���� D. �ų�126 kJ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com