
���� һ����˵�����ý�����ǽ����γ����Ӽ����ǽ���֮���γɹ��ۼ�����ͬ�ǽ���֮���γɼ��Թ��ۼ���ͬ�ַǽ���֮���γɷǼ��Թ��ۼ��������Ӽ���һ��Ϊ���Ӿ��壬��ԭ�ӹ����Һ����ۼ���Ϊԭ�Ӿ��壬�Դ������
��� �⣺NaOH�����Ӽ���O-H���Լ���Ϊ���Ӿ��壻
Na2Sֻ�����Ӽ���Ϊ���Ӿ��壻
H2O2��O-H���Լ���O-O�Ǽ��Լ���Ϊ���Է��ӣ����ڷ��Ӿ��壻
H2Oֻ��O-H���Լ���Ϊ���Է��ӣ����ڷ��Ӿ��壻
Na2O2�����Ӽ���O-O�Ǽ��Լ���Ϊ���Ӿ��壻
C2H2��C-H���Լ���C��C�Ǽ��Լ����ҽṹ�Գƣ�Ϊ�Ǽ��Է��ӣ����ڷ��Ӿ��壻
SiC���庬Si-C���Լ�����ԭ�ӹ��ɣ�Ϊԭ�Ӿ��壬��
��1������ֻ�������Ӽ������Ӿ�����Na2S���ʴ�Ϊ��Na2S��
��2�����мȺ������Ӽ��ֺ��м��Թ��ۼ������Ӿ�����NaOH���ʴ�Ϊ��NaOH��
��3�����мȺ������Ӽ��ֺ��зǼ��Թ��ۼ������Ӿ�����Na2O2���ʴ�Ϊ��Na2O2��
��4�����к��м��Թ��ۼ��ͷǼ��Թ��ۼ��ķǼ��Է�����C2H2���ʴ�Ϊ��C2H2��
��5�����к��м��Թ��ۼ��ͷǼ��Թ��ۼ��ļ��Է�����H2O2���ʴ�Ϊ��H2O2��
��6�����к��м��Թ��ۼ���ԭ�Ӿ�����SiC���ʴ�Ϊ��SiC��
���� ���⿼�黯ѧ�����������ͣ�Ϊ��Ƶ���㣬���ջ�ѧ�����γɼ��жϵ�һ����ɡ�����Ĺ�����Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬ע��ۼ������ͣ���Ŀ�ѶȲ���


 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
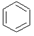 ��b����
��b����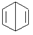 ��d����
��d���� ��p����˵����ȷ���ǣ�������
��p����˵����ȷ���ǣ�������| A�� | b��d��p��ͬ���칹�� | |
| B�� | b��d��p�Ķ��ȴ����ֻ������ | |
| C�� | b��d��p���������Ը��������Һ��Ӧ | |
| D�� | b��d��p��ֻ��b������ԭ�Ӵ���ͬһƽ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ۻ������ڲ����������Ӽ� | |
| B�� | ԭ�ӻ����Ӽ�������л�ѧ�� | |
| C�� | ȫ���ɷǽ���Ԫ����ɵĻ����ﲻһ���ǹ��ۻ����� | |
| D�� | ��IA����Ԫ�����VIIAԪ��һ���γ����Ӽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
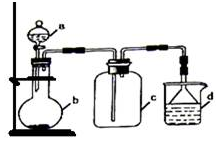 ʵ������ijЩ�������ȡ���ռ���β������װ����ͼ��ʾ��ʡ���˾���װ�ã������ô�װ�úͱ����ṩ������������ʵ�飬�������ѡ���ǣ�������
ʵ������ijЩ�������ȡ���ռ���β������װ����ͼ��ʾ��ʡ���˾���װ�ã������ô�װ�úͱ����ṩ������������ʵ�飬�������ѡ���ǣ�������| ѡ�� | a�е����� | b�е����� | c���ռ������� | d�е����� |
| A | Ũ��ˮ | CaO | NH3 | H2O |
| B | Ũ���� | Na2SO3 | SO2 | NaOH��Һ |
| C | ϡ���� | Cu | NO2 | H2O |
| D | Ũ���� | MnO2 | Cl2 | NaOH��Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ԭ�������ͭԪ�ص�������һ��Ϊ0.64g | |
| B�� | �����������Ϊ2.016L | |
| C�� | ʵ���з�Ӧ����������ʵ���Ϊ0.1mol | |
| D�� | ԭ�������������������Ϊ78.5% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����¶� | |
| B�� | ����������������䣬����A��g�������ʵ��� | |
| C�� | ����������ѹǿ���䣬���뺤�� | |
| D�� | ����������������䣬���뺤�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�22.4 L CO��C2H4���������ܷ�����Ϊ2NA������Ϊ28 g | |
| B�� | ���³�ѹ�£�1 mol����-CH3������������Ϊ9NA | |
| C�� | 1 L 0.1 mol/L ��NH4��2SO4��Һ�к�NH4+������Ϊ0.2NA | |
| D�� | �ö��Ե缫��ⱥ��ʳ��ˮ�����������ռ�������4.48Lʱ�����·��ת�Ƶ�����Ϊ0.2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ͬ��������ͬ�ܶȵ�N2��CO | B�� | ͬ�¶ȡ�ͬ�����N2��H2 | ||
| C�� | ͬ�����ͬ�ܶȵ�C2H4��CH4 | D�� | ͬѹǿ��ͬ�����N2O��CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ҽ���Һ�м������ᡢ˫��ˮ��2I-+2H++H2O2�TI2+2H2O | |
| B�� | ������������ˮ����ˮ��Cl2+H2O?H++Cl-+HClO | |
| C�� | ��Al2��SO4��3��Һ�м�������İ�ˮ��Al3++4NH3•H2O�TAlO2-+4NH4++2H2O | |
| D�� | ���ˮ�еμӱ���FeCl3�ƽ��壺Fe3++3H2O?Fe ��OH��3�����壩+3H+ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com