
��10�֣�������ͼ��ʾװ����ȡ������������������գ�
(1)�Թ�a�������Ũ���ᡢ��������Ҵ���2 mL����ȷ�ļ���˳������___________________________________
(2)Ϊ��ֹa�е�Һ����ʵ��ʱ�������У����ڼ���ǰ��ȡ�Ĵ�ʩ��__________��
(3)ʵ���м����Թ�a��Ŀ���ǣ�
��_________________________________________��
��__________________________________________;
(4)������ͬλ��ʾ�ٷ���ȷ����Ӧ��ϼ�����������������е�����18O���Ҵ��е�����16Oд���ܱ�ʾ��ȡ���������ϼ�����Ļ�ѧ����ʽ��__________________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
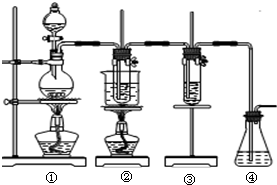 ��ʵ�����������ͼ��ʾװ����ȡ����غ�̽����ˮ�����ʣ� ͼ�У���Ϊ��������װ�ã��ڵ��Թ���ʢ��15mL 30%KOH��Һ��������ˮԡ�У��۵��Թ��������ɫʯ����Һ����Ϊβ������װ�ã�����д���пհף�
��ʵ�����������ͼ��ʾװ����ȡ����غ�̽����ˮ�����ʣ� ͼ�У���Ϊ��������װ�ã��ڵ��Թ���ʢ��15mL 30%KOH��Һ��������ˮԡ�У��۵��Թ��������ɫʯ����Һ����Ϊβ������װ�ã�����д���пհף� ͼ�з��ϸþ����ܽ�����ߵ���
ͼ�з��ϸþ����ܽ�����ߵ���| ʵ������ | ԭ�� |
| ��Һ�������ɫ��Ϊ ��ɫ ��ɫ ɫ |
������ˮ��Ӧ���ɵ�H+ʹʯ���ɫ |
| �����Һ��Ϊ��ɫ | ������ˮ��Ӧ����HClO����Ư���� ������ˮ��Ӧ����HClO����Ư���� |
| Ȼ����Һ����ɫ��Ϊ dz����ɫ dz����ɫ ɫ |
���������ܽ���ˮ�� ���������ܽ���ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ �� | �й���Ϣ |
| A | ����������Ӧ��ˮ����ף���������̬�⻯��ң���Ӧ������ |
| B | �����������Ǵ�����������2�� |
| C | M������3������ |
| D | ������ԭ�Ӱ뾶��������Ԫ�� |
| E | �������������۴�����Ϊ6 |

 A2O4��g������H��0���ں��º��������£���һ����AO2��A2O4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��t�ı仯��ϵ����ͼ��ʾ��
A2O4��g������H��0���ں��º��������£���һ����AO2��A2O4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��t�ı仯��ϵ����ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ����ѧ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��14�֣���ʵ�����������ͼ��ʾװ����ȡ����ء��������ƺ�̽����ˮ�����ʡ�
ͼ�У���Ϊ��������װ�ã��ڵ��Թ���ʢ��15 mL 30% KOH��Һ���������� ˮԡ�У��۵��Թ���ʢ��15 mL 8% NaOH��Һ�������ڱ�ˮԡ�У��ܵ��Թ��������ɫʯ����Һ����Ϊβ������װ�á�
ˮԡ�У��۵��Թ���ʢ��15 mL 8% NaOH��Һ�������ڱ�ˮԡ�У��ܵ��Թ��������ɫʯ����Һ����Ϊβ������װ�á�
����д���пհף�
�� ��ȡ����ʱ������ƿ�����һ�����Ķ������̣�ͨ��________����д�������ƣ�����ƿ�м���������Ũ���ᡣʵ������Cl2�Ļ�ѧ����ʽ ��ʵ��ʱΪ�˳�ȥ�����е�HCl���壬���ڢ����֮�䰲װʢ��_______����д���б����ĸ���ľ���װ�á�
| A����ʯ�� | B������������Һ | C������ʳ��ˮ | D��Ũ���� |
 06 mol��
06 mol�� ����Ҫԭ���У���_______________________________________________��
����Ҫԭ���У���_______________________________________________��
 дʵ��������ƣ���
дʵ��������ƣ���ʵ�� ���� ���� | ԭ�� |
| ��Һ�������ɫ��Ϊ_______ɫ | ������ˮ��Ӧ ���ɵ�H+ʹʯ���ɫ ���ɵ�H+ʹʯ���ɫ |
| �����Һ��Ϊ��ɫ | ____________________________ __________ __________ |
| Ȼ����Һ����ɫ��Ϊ_______ɫ | ______________________ ________________ ________________ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��ɽ��ʡ�����и���3�½�ѧ������⣨һģ����ѧ�Ծ��������棩 ���ͣ�ѡ����
�����й�ʵ���������ȷ����

A���������Ż�ʱ��������մˮ��ë������
B��ʵ���ҿ�����ͼ��ʾװ����ȡ���Ͱ�ˮ
C����������Һ�����ڴ�������ϸ��ƿ��
D��������ƿ������Һ������ʱ���ӿ̶��ߣ�������ҺŨ��ƫС
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��ʵ�����������ͼ��ʾװ����ȡ����ء��������ƺ�̽����ˮ�����ʡ�

ͼ�У���Ϊ��������װ�ã��ڵ��Թ���ʢ��15 mL 30% KOH��Һ����������ˮԡ�У��۵��Թ���ʢ��15 mL 8% NaOH��Һ�������ڱ�ˮԡ�У��ܵ��Թ��������ɫʯ����Һ����Ϊβ������װ�á�
����д���пհף�
����ȡ����ʱ������ƿ�����һ�����Ķ������̣�ͨ��________����д�������ƣ�����ƿ�м���������Ũ���ᡣʵ������Cl2�Ļ�ѧ����ʽ ��ʵ��ʱΪ�˳�ȥ�����е�HCl���壬���ڢ����֮�䰲װʢ��_______����д���б����ĸ���ľ���װ�á�
A����ʯ�� B������������Һ C������ʳ��ˮ D��Ũ����
�� �������������������20 mL 12 mol��L��1��Ũ�����ϼ��ȣ���ַ�Ӧ�����ɵ�������������0. 06 mol������Ҫԭ���У�
��______________________________________ _________��
��__________________________________________________________________________��
�� �Ƚ���ȡ����غʹ������Ƶ����������ߵIJ�����
�� ��
�� ��
��4����Ӧ��Ͼ���ȴ�ڵ��Թ����д�������������
����ͼ�з��ϸþ����ܽ�����ߵ���_______����д�����ĸ����
�ڴӢڵ��Թ��з�����þ���ķ�����__________����дʵ��������ƣ���
�� ʵ���пɹ۲쵽�ܵ��Թ�����Һ����ɫ���������±仯������д�±��еĿհס�
|
ʵ������ |
ԭ�� |
|
��Һ�������ɫ��Ϊ_______ɫ |
������ˮ��Ӧ���ɵ�H+ʹʯ���ɫ |
|
�����Һ��Ϊ��ɫ |
______________________________________ |
|
Ȼ����Һ����ɫ��Ϊ_______ɫ |
______________________________________ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com