
�� �� ����ʽ��HCl ��Է���������36.5 ��ۺϸ� �ܶ�Լ�� HCl������������36.5% ����GB622-89 �Լ���������֤��ţ� |
(1)����������ʵ���Ũ��Ϊ���٣�(��ʽ����)
(2)ȡ������25.4 mL��2.00 mol��L-1������������Һ100 mL��ϣ��ٽ���Ϻ���Һϡ����
 �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д� �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼΪij���������Լ�ƿ��ǩ�ϵIJ������ݡ��ʣ�
| �� �� ����ʽ��HCl ��Է���������36.5 ��ۺϸ� �ܶ�Լ��1.18 g/cm3 HCl������������36.5% ����GB622~89 �Լ���������֤��ţ� |
��1������������ʵ���Ũ��Ϊ���٣�����ʽ���㣩
��2��ȡ������25.5 mL��2.00 mol/L������������Һ100 mL��ϣ��ٽ���Ϻ���Һϡ����1.00 L����ʱ��Һ��pHԼΪ���٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ��������ѧ������һ���¿����أ���ѧ�Ծ����������� ���ͣ������
��12�֣���1����ͼΪij���������Լ�ƿ��ǩ�ϵIJ������ݣ��ʣ�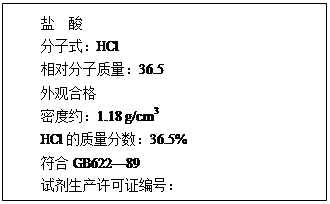
����������ʵ���Ũ��Ϊ���٣�(��ʽ����)
(2) ��֪п�������ǻ��ý����������������������ǿ�ᣬ��������ǿ������������������ڰ�ˮ����������п�����ڰ�ˮ������Zn��NH3��42+����ش��������⣺
��1����������������������Һ����Һ����Ԫ�صĴ�����ʽΪ ���û�ѧʽ��ʾ����
��2��д��п������������Һ��Ӧ�Ļ�ѧ����ʽ ��
��3�����и����е�������Һ������μӵ�ʵ�鷽�����ɼ������ ��
������������������ ���������Ͱ�ˮ
������п���������� ������п�Ͱ�ˮ
��4��д�������������백ˮ��Ӧ�����ӷ���ʽ .��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ������һ���¿����أ���ѧ�Ծ��������棩 ���ͣ������
��12�֣���1����ͼΪij���������Լ�ƿ��ǩ�ϵIJ������ݣ��ʣ�
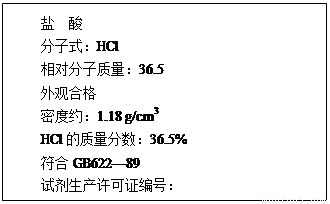
����������ʵ���Ũ��Ϊ���٣�(��ʽ����)
(2) ��֪п�������ǻ��ý����������������������ǿ�ᣬ��������ǿ������������������ڰ�ˮ����������п�����ڰ�ˮ������Zn��NH3��42+����ش��������⣺
��1����������������������Һ����Һ����Ԫ�صĴ�����ʽΪ ���û�ѧʽ��ʾ����
��2��д��п������������Һ��Ӧ�Ļ�ѧ����ʽ ��
��3�����и����е�������Һ������μӵ�ʵ�鷽�����ɼ������ ��
������������������ ���������Ͱ�ˮ
������п���������� ������п�Ͱ�ˮ
��4��д�������������백ˮ��Ӧ�����ӷ���ʽ .��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ������ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��5�֣���ͼΪij���������Լ�ƿ��ǩ�ϵIJ������ݡ��ʣ�

��1������������ʵ���Ũ��Ϊ���٣�
��2��ȡ������25.4 mL��2.00 mol��L-1����������
��Һ100 mL��ϣ��ٽ����Һϡ�͵�1.00 L��
��ʱ��Һ��pHԼΪ���٣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com