
��ͼ��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��109g5.51����NaOH��Һ��������CuSO4��Һ��200g10.00����K2SO4��Һ���缫��Ϊʯī�缫��
��ͨ��Դ������һ��ʱ���ñ���K2SO4Ũ��Ϊ10.47��������c�缫�������ӡ��ݴ˻ش����⣺
��1���缫b�Ϸ����ĵ缫��ӦΪ___________________________________��
��2���缫b�����ɵ������ڱ�״���µ����Ϊ__________________����ʱ���ձ���NaOH��Һ�����ʵ���Ũ��Ϊ������Һ���ܶ�Ϊ1g/cm3��_______________��
��3���缫c�������仯��___________g����ʹ�������еĵ��Һ�ָ�����ʼ״̬��Ӧ������Һ�м���������___________������ĸ��ţ���
| A��Cu(OH)2 | B��Cu2O | C��CuCO3 | D��Cu2(OH)2CO3 |
��1�� 4OH���� 4e���� 2H2O + O2����2�֣�
��2��5.6 L��2�֣� 1.5mol/L��2�֣�
(3)32��2�֣� C��1�֣�
(4)��ͭ��ͭ��1�֣� ��ͭ��1�֣�
���������������1����������c�缫�������ӿ�֪c���Ϊ��������M�缫Ϊ��Դ�ĸ�����N�缫Ϊ������b�缫Ϊ����������OH?ʧȥ���ӵķ�Ӧ��4OH���� 4e���� 2H2O + O2����
��2������200g10.00����K2SO4��ҺŨ�ȱ�Ϊ10.47���������H2O������Ϊ200g -200g��10.00����10.47��=9g�����H2Oʱ��ת�Ƶ��ӵĶ�Ӧ��ϵΪ��H2O~2e?����n(e?)=2��9g��18g/mol=1mol�����ݵ缫b������Ӧ��4OH���� 4e���� 2H2O + O2������������ɵ����壺V(O2)=1mol��4��22.4L/mol=5.6L�����ձ���NaOH��ҺҲ�����9gH2O����Һ���Ϊ����109g-9g����1000g/L=0.1L��c(NaOH)=109g��5.51%��40g/mol��0.1L=1.5mol?L?1��
��3���缫cΪ������������Ӧ��Cu2++2e?=Cu�����ɵ�Cu����Ϊm(Cu)=1mol��2��64g/mol=32g���������H2SO4��O2��Cu�����ݳ���ʲô��ʲô��ԭ����CuCO3���ʹ�������еĵ��Һ�ָ�����ʼ״̬��
��4����⾫��ͭ����ͭ���������缫��ͭ����������c�缫�IJ���Ϊ��ͭ��d�缫�IJ���Ϊ��ͭ��
���㣺���⿼��缫����ʽ����д��������ļ��㡢�缫���ϵ�ѡ�������Һ�ظ��������ʵ�ѡ��


 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��.������ʳ����ȷ��ҩ�������彡������Ҫ��֤��
����������������Aʳ�Σ�BС�մ�Cƻ��֭��D�����ǣ�E��ù�أ��밴����Ҫ�����(�����)��
����ά����C���� ����ֱ�ӽ���ѪҺ�������������� ��Ӧ����㷺�Ŀ�����֮һ���� ���ȿ���Ϊ���ɼ����ֿ�����θ�������� ��ʳ�ù��������Ѫѹ���ߡ���������� ��
��.������������ʹ�����������������������һ����Ҫ��־��
��1��д����ҵ���ó�������������Ҫ��ѧ��Ӧ����ʽ�� ��
��2�������˵�����δ��ʱϴ������Һ�к�NaCl��,�ڶ�������ʴ���ֺ��ɫ��ߡ��Իش�
�������ĸ�ʴ��Ҫ���� ���ѧ��绯ѧ����ʴ��ɵġ��γɵ��������Ҫ�ɷ��� ��
��Ϊ��ֹ�ִ��Ĵ����ں�ˮ�и�ʴ��һ���ڴ������� ���п�顱��ͭ�顱����
��.������������������ͷ�չ����Ҫ���ʻ���������ʹ�ò��Ͽ��Ը������ǵ����
��1���������ݽ���������������ϡ����в��ϲ����ڹ����β��ϵ��� ������ĸ����
A��ʯ��ʯ B��ˮ�� C������
��2�������в����У��������ǽ������ϵ��� (����ĸ)������������Ʒ���� ��
A�����ڡ������� B��������ϩ���ϡ���C���������մ� D��������
��3�������йغϽ����ʵ�˵����ȷ���� ������ĸ����
A���Ͻ���۵�һ������ijɷֽ�����
B���Ͻ��Ӳ��һ������ijɷֽ�����
C����ɺϽ��Ԫ��������ͬ���Ͻ�����ܾ�һ����ͬ
D���Ͻ�����ɷֽ�����ȣ�����������������������ѧ���е����
��4���ϳ����ϡ��ϳ��� �dz�˵������ϳɲ��ϡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ���������ͼ��ʾ��װ�á���֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH=2K2CO3+6H2O����ش�
��ͨ��O2�ĵ缫������ ��B�缫�������� ��
��ͨ��CH3OH�ĵ缫�ĵ缫��Ӧʽ�� ��A�缫�ĵ缫��ӦʽΪ ��
���ҳ����Ϊ1L����AgNO3����������µ��һ��ʱ�����Һ��PH��Ϊ1���������ʱ����ת�Ƶĵ�����ĿΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������һ�ֿ��Է�����硢�ŵ��װ�á���һ�������ڳ��ͷŵ�ʱ�����ķ�ӦΪNiO2+Fe+2H2O Fe(OH)2+Ni(OH)2
Fe(OH)2+Ni(OH)2
��1�������طŵ�ʱ��������ԭ��Ӧ�������� ������ĸ����ͬ����
A��NiO2 B��Fe C��Fe(OH)2 D��Ni(OH)2
��2�������йظõ�ص�˵������ȷ����
A���ŵ�ʱ�������Һ��ǿ����
B���ŵ�ʱ5.6g Feȫ��ת��ΪFe(OH)2ʱ�����·��ͨ����0.2 mol����
C�����ʱ������ӦΪNi(OH)2+2OH��?2e��==NiO2+2H2O
D�����ʱ����������Һ�ļ��Ա��ֲ���
��3���ô����ص�⺬��0.01 mol CuSO4��0.01 mol NaCl�Ļ����Һ100 mL�����صĵ缫��Ϊ���Ե缫������Һ�е�Cu2+ ȫ��ת����Cuʱ�����������������ڱ�״���µ����Ϊ �����������Һ��ˮϡ����1L����ʱ��Һ��pH= ��
��4���ô����ؽ��е�⣬�ҵ��صĵ缫��Ϊͭ�缫���������ҺΪŨ��Һ��NaCl��Һ�Ļ��Һ�����һ��ʱ���ͬѧ�Ǿ���ط��֣�������������������ɫ�������������ɺ�ɫ�������������ϵ�֪�ú�ɫ������Cu2O��д���������ϵĵ缫��Ӧʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������ԭ��Ӧ��2Ag+(aq)+Cu(s)=Cu2+(aq)+2Ag(s)��Ƶ�ԭ�������ͼ���ش��������⣺
��1���缫X�IJ����� ���������ҺY�� ��
��2�����缫Ϊ��ص� ����
��3���������������� �ƶ������ �����ҡ�����
��4�����·�е����Ǵ� ����缫������������ͬ���缫���� �缫��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ҫ����գ�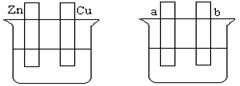
A B
��1����Aͼ�У�ϡ����Ϊ�������Һ���õ������Ӻ�ͭƬ�缫��Ӧʽ ��
��2����Bͼ�����ֱ����Դ����Ҫ��a����ͭ�����Ա�Ҫ�����Ӻ�װ�ý� ��b���缫��Ӧʽ ��
��3����Bͼ�����ֱ����Դ�����缫Ϊ���Ե缫���������Һ��CuSO4��Һ��������������ܷ�Ӧ���ӷ���ʽΪ ����������3.2 g���������Ϸų��������ڱ�״���µ������_____L������һ������ ���ѧʽ������Һ�ָܻ�������ǰ��ȫһ�¡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ�Ƿḻ����Դ���⣬�Ӻ�ˮ����ȡԪ���ǻ�ѧ��ҵ����Ҫ��ɲ��֡�
��1�����ξ��ƾ��dz�ȥ���е�Ca2+��Fe3+��SO42-����ɳ�����ʣ��������Լ��У���Na2CO3��Һ ��HCl�����ᣩ ��Ba��OH��2��Һ���������Լ�������˳����_________������ţ���
��2�����������С���ˮ���塱����ȡ�嵥�ʷ�Ӧ�����ӷ���ʽΪ��__________��
��3��ijͬѧ�������ͼװ�ý������µ绯ѧʵ�顣
�ٵ�����K��a����ʱ�������������ݲ�����������Ϊ_______����
��һ��ʱ���ʹ����K��a�Ͽ�����b����ʱ�����߿��ڵ�װ�ÿɳ�Ϊ__________����д����ʱFe�缫�ϵĵ缫��Ӧʽ_________________��
��4��ij������ʢ��CaSO4����Һ�ķ�Ӧ����ͨ�백������ȡ���ʣ�NH4��2SO4��Ч�����á���ͨ��CO2��������������NH4��2SO4���������ԭ�� ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͼװ���У�b�缫�ý��� M�Ƴɣ�a��c��dΪʯī�缫����ͨ��Դ������M������b����ͬʱa��d�缫�ϲ������ݡ��Իش�
��1��aΪ ����c���ĵ缫��ӦʽΪ ��
��2��������һ��ʱ�������c���ϵ��Թ���Ҳ���ռ��������壬��ʱc���ϵĵ缫��ӦʽΪ ��
��3����d�����ռ���44.8mL���壨��״����ʱֹͣ��⣬a���Ϸų�����������ʵ���Ϊ ����b�缫�ϳ�������M������Ϊ0.432g����˽�����Ħ������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
�����̿�MnCO3��������Fe2O3��FeO��HgCO3��2HgO�����ʣ���ҵ�������̿���ȡ�̣������������£�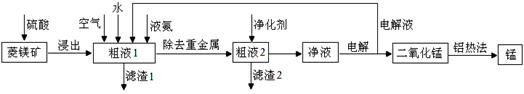
��ش��������⣺
��1�����Һ1�м����ˮ�����Ҫ �������ܴﵽ����Ҫ��
��2���������õĿ�������Ĥ���뷨�Ʊ��ĸ����������÷�����ԭ���� ��
��3����������Ҫ�ɷ�Ϊ(NH4)2S����Һ2�з�����Ҫ��Ӧ�����ӷ���ʽΪ ��
��4��д�������ĵ缫��Ӧʽ ��˵�����Һѭ����ԭ�� ��
��5��д�����ȷ����̵Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com