
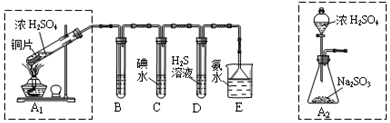
·ÖĪö £Ø1£©ĶŗĶÅØĮņĖį¼ÓČČ·“Ӧɜ³É¶žŃõ»ÆĮņĘųĢ壬B”¢C”¢D·Ö±šÓĆÓŚ¼ģŃéSO2µÄĘư׊Ō”¢»¹ŌŠŌŗĶŃõ»ÆŠŌ£®ĘäÖŠC”¢D·Ö±šĪŖµāĖ®ŗĶĮņ»ÆĒāµÄĖ®ČÜŅŗ£¬¼ģŃ鶞Ńõ»ÆĮņĘư׊ŌæÉŅŌĶعżĘ·ŗģŹŌŅŗ£»¶žŃõ»ÆĮņ¾ßÓŠ»¹ŌŠŌ£¬ŗĶµāµ„ÖŹ·“Ӧɜ³ÉĮņĖįŗĶµā»ÆĒā£»
£Ø2£©ŅĄ¾Ż×°ÖĆĶ¼¶Ō±Č·ÖĪö²»Ķ¬£¬×ܽįÓÅµć£®
½ā“š ½ā£ŗ£Ø1£©ŅĄ¾Ż×°ÖĆĶ¼æÉÖŖ£¬ĶŗĶÅØĮņĖį¼ÓČČ·“Ӧɜ³É¶žŃõ»ÆĮņĘųĢ壬B”¢C”¢D·Ö±šÓĆÓŚ¼ģŃéSO2µÄĘư׊Ō”¢»¹ŌŠŌŗĶŃõ»ÆŠŌ£®ĘäÖŠC”¢D·Ö±šĪŖµāĖ®ŗĶĮņ»ÆĒāµÄĖ®ČÜŅŗ£¬C¼ģŃ鶞Ńõ»ÆĮņµÄ»¹ŌŠŌ£¬D¼ģŃ鶞Ńõ»ÆĮņµÄŃõ»ÆŠŌ£¬¼ģŃ鶞Ńõ»ÆĮņĘư׊ŌæÉŅŌĶعżĘ·ŗģŹŌŅŗ£¬Ę·ŗģĶŹÉ«ŹĒ¶žŃõ»ÆĮņĘųĢåµÄĢŲŠŌ£»¶žŃõ»ÆĮņ¾ßÓŠ»¹ŌŠŌ£¬ŗĶµāµ„ÖŹ·“Ӧɜ³ÉĮņĖįŗĶµā»ÆĒā£»·“Ó¦µÄĮ½ÖÖ·½³ĢŹ½ĪŖ£ŗSO2+I2+2H2OØTSO42-+2I-+4H+£»
¹Ź“š°øĪŖ£ŗĘ·ŗģČÜŅŗ£» SO2+I2+2H2OØTSO42-+2I-+4H+£»
£Ø2£©×°ÖĆĶ¼·ÖĪöæÉÖŖ£¬A2µÄÖĘČ”×°ÖĆĄ““śĢęA1×°ÖĆ£¬æÉŅŌ²»ŠčŅŖ¼ÓČČ»ņ½ŚŌ¼ÄÜŌ“»ņŅ©Ę·£¬ÓĆ·ÖŅŗĀ©¶·¼ÓČėĮņĖįæÉŅŌæŲÖĘ·“Ó¦ĖŁĀŹ£¬Ņ×ÓŚæŲÖĘ·“Ó¦½ųŠŠ£»»ņ·“Ó¦øü³ä·Ö£¬¹Ź“š°øĪŖ£ŗ²»ÓĆ¼ÓČČ»ņ½ŚŌ¼ÄÜŌ“»ņŅ©Ę·£»»ņĻą¶Ō°²Č«£»»ņŅ×ÓŚæŲÖĘ·“Ó¦½ųŠŠ£»»ņ·“Ó¦øü³ä·Ö£®
µćĘĄ ±¾Ģāæ¼²éĮĖ¶žŃõ»ÆĮņŠŌÖŹµÄŹµŃéŃéÖ¤·½·ØŗĶŹµŃéÉč¼Ę·ÖĪö²½Öč£¬ÕĘĪÕŹµŃ黳“”ŗĶĄė×ÓŠŌÖŹ£¬ÕżČ·Ń”ŌńŹŌ¼ĮĢāÄæÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
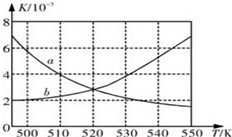 ¢ń£ŗĪŖĮĖ¼õÉŁCOµÄÅÅ·Å£¬Ä³»·¾³ŃŠ¾æŠ”×éŅŌCOŗĶH2 ĪŖŌĮĻŗĻ³ÉĒå½ąÄÜŌ“¶ž¼×ĆŃ£ØDME£©£¬·“Ó¦ČēĻĀ£ŗ4H2£Øg£©+2CO£Øg£©
¢ń£ŗĪŖĮĖ¼õÉŁCOµÄÅÅ·Å£¬Ä³»·¾³ŃŠ¾æŠ”×éŅŌCOŗĶH2 ĪŖŌĮĻŗĻ³ÉĒå½ąÄÜŌ“¶ž¼×ĆŃ£ØDME£©£¬·“Ó¦ČēĻĀ£ŗ4H2£Øg£©+2CO£Øg£©| Ź±¼ä/min | 0 | 20 | 40 | 80 | 100 |
| n£ØH2£©/mol | 2.0 | 1.4 | 0.85 | 0.4 | - |
| n£ØCO£©/mol | 1.0 | - | 0.425 | 0.2 | 0.2 |
| n£ØCH3OCH3£©/mol | 0 | 0.15 | - | - | 0.4 |
| n£ØH2O£©/mol | 0 | 0.15 | 0.2875 | 0.4 | 0.4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
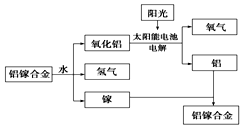 ĆĄ¹śĘÕ¶Č“óѧъ¾ææŖ·¢³öŅ»ÖÖĄūÓĆĀĮļŲŗĻ½šÖʱøĒāĘųµÄŠĀ¹¤ŅÕ£ØČēĶ¼ĖłŹ¾£©£®ĻĀĮŠÓŠ¹ŲøĆ¹¤ŅÕµÄĖµ·Ø“ķĪóµÄŹĒ£Ø””””£©
ĆĄ¹śĘÕ¶Č“óѧъ¾ææŖ·¢³öŅ»ÖÖĄūÓĆĀĮļŲŗĻ½šÖʱøĒāĘųµÄŠĀ¹¤ŅÕ£ØČēĶ¼ĖłŹ¾£©£®ĻĀĮŠÓŠ¹ŲøĆ¹¤ŅÕµÄĖµ·Ø“ķĪóµÄŹĒ£Ø””””£©| A£® | ĀĮļŲŗĻ½šÓėĖ®·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ2Al+3H2O$\frac{\underline{\;Ņ»¶ØĢõ¼ž\;}}{\;}$Al2O3+3H2”ü | |
| B£® | ×Ü·“Ó¦Ź½ĪŖ2H2O$\frac{\underline{\;Ņ»¶ØĢõ¼ž\;}}{\;}$2H2”ü+O2”ü | |
| C£® | øĆ¹ż³ĢÖŠ£¬ÄÜĮæµÄ×Ŗ»ÆŠĪŹ½Ö»ÓŠĮ½ÖÖ | |
| D£® | ĀĮļŲŗĻ½šæÉŅŌŃ»·Ź¹ÓĆ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | ¢Ś¢Ü | B£® | ¢Ś¢Ü¢Ż | C£® | ¢Ś¢Ü¢Ż¢Ž | D£® | ¢Ū¢Ü¢Ż¢Ž |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¼ÓČČ”¢ĶØĒāĘų”¢Ķ£Ö¹¼ÓČČ”¢¼ĢŠųĶØĒāĘųÖĮŹŌ¹ÜĄäČ“ | |
| B£® | ĶØĒāĘų”¢¼ÓČČ”¢Ķ£Ö¹¼ÓČČ”¢¼ĢŠųĶØĒāĘųÖĮŹŌ¹ÜĄäČ“ | |
| C£® | ĶØĒāĘųŗóĮ¢¼“µćČ¼¾Ę¾«µĘ¼ÓČČ | |
| D£® | Ķ£Ö¹¼ÓČČŗóĮ¢¼“Ķ£Ö¹ĶØĒāĘų |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 11.2 L CO2Ėłŗ¬·Ö×ÓŹżĪŖ0.5 NA | |
| B£® | 32gO2ŗĶO3µÄ»ģŗĻĘųĢåÖŠŗ¬ÓŠµÄŃõŌ×ÓŹżĪŖ2NA | |
| C£® | ±źæöĻĀ£¬11.2LŅŅ“¼£ØC2H5OH£©ÖŠĖłŗ¬µÄĢ¼Ō×ÓŹżÄæĪŖNA | |
| D£® | 14.2 g Na2SO4¹ĢĢåÖŠŅõĄė×ÓĖł“ųµēŗÉŹżĪŖ0.1NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 2Al2O3+3C$\frac{\underline{\;øßĪĀ\;}}{\;}$4Al+3CO2”ü | B£® | CuCl2$\frac{\underline{\;µē½ā\;}}{\;}$Cu+Cl2”ü | ||
| C£® | Fe3O4+4CO$\frac{\underline{\;øßĪĀ\;}}{\;}$3Fe+4CO2 | D£® | 2HgO$\frac{\underline{\;µē½ā\;}}{\;}$2Hg+O2”ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĆĢøÖ | B£® | ĒąĶ | C£® | ĀĮļ®ŗĻ½š | D£® | ļēÄų“¢ĒāŗĻ½š |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com