
ij��ɫ��Һ����Na+��Ag+��Ba2+��Al3+��AlO2����MnO4����CO32����SO42���е���������ɡ�ȡ����Һ��������ʵ�飺��1��ȡ������Һ������������ᣬ���������ɣ����õ���ɫ��Һ����2���ڣ�1��������Һ�м������NH4HCO3��Һ�����������ɣ�ͬʱ������ɫ�����ף���3���ڣ�2��������Һ�м������Ba(OH)2��ҺҲ���������ɣ�ͬʱ������ɫ�����ҡ�������������ԭ��Һ��һ�����ڵ���
A��SO42-��AlO2����Na+ B��Na+��CO32����AlO2��
C��CO32����Na+��Al3+ D��MnO4����Na+��CO32��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�������������и�һ6���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
X��Y��Z��W��R���ڶ���������Ԫ�ء�X��ԭ�Ӱ뾶�Ƕ���������Ԫ�������ģ�YԪ�ص�ԭ������������Ϊm������������Ϊn��ZԪ�ص�ԭ��L�������Ϊm��n��M�������Ϊm��n��WԪ����ZԪ��ͬ���壬RԪ��ԭ����YԪ��ԭ�ӵĺ��������֮��Ϊ2:1�� ���������������
A. X��Y�γɵ����ֻ����������������ӵĸ����Ⱦ�Ϊ1��2
B. Y���⻯���R���⻯���ȶ����۷е��
C. Z��W��R����������Ӧˮ���������ǿ��˳���ǣ�R>W>Z
D. RY2��WY2ͨ��Ba��NO3��2��Һ�о��ް�ɫ�������ɡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�������������и߶�6��С��ɹ����ջ�ѧ���������棩 ���ͣ�ѡ����
��NA��ʾ�����ӵ�������ֵ������������ȷ���ǣ� ��
A�����³�ѹ�µ�33.6L������27g����ַ�Ӧ��ת�Ƶ�����С��3NA
B��0.1 mol Cl2��ȫ����ˮ��ת�Ƶĵ�����ĿΪ0.1NA
C��56g��ƬͶ������ŨH2SO4������NA��SO2����
D��5NH4NO3 2HNO3+4N2��+9H2O��Ӧ�У�����28gN2ʱ��ת�Ƶĵ�����ĿΪ3.25NA
2HNO3+4N2��+9H2O��Ӧ�У�����28gN2ʱ��ת�Ƶĵ�����ĿΪ3.25NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ������ʦ���и�һ��ѧ��6���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�����£�ij��Ӧ��ƽ�⣬ƽ�ⳣ��K=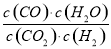 ������ʱ���¶����ߣ�H2Ũ�ȼ�С������˵����ȷ����
������ʱ���¶����ߣ�H2Ũ�ȼ�С������˵����ȷ����
A���÷�Ӧ���ʱ�Ϊ��ֵ
B�����º����£�����ѹǿ��H2Ũ��һ����С
C�������¶ȣ��淴Ӧ���ʼ�С
D���÷�Ӧ��ѧ����ʽΪCO+H2O=CO2+H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�������ĵ����һ�и߶�6���¿���ѧ�Ծ��������棩 ���ͣ������
1Lij�����Һ�����ܺ��е��������±���

��1��������Һ����μ���NaOH��Һ���ʵ����ȣ�������������������ʵ���(n)�����NaOH��Һ�����(V)�Ĺ�ϵ��ͼ��ʾ��
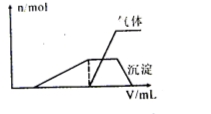
�������Һ��һ�����е������� ��
�ڿ��ܴ��ڵ��������� ��
�ۿ϶������ڵ��������� ��
��2������⣬����Һ�л����д�����Cl����Br����I��������2 L�û����Һ��ͨ��һ������Cl2����Һ��Cl����Br����I�������ʵ�����ͨ��Cl2�����(��״��)�Ĺ�ϵ�����ʾ��������ش��������⡣

��a��ֵΪ ��
�ڵ�ͨ��Cl2�����Ϊ3.36 L����״̬�£�ʱ����Һ�з�����Ӧ�����ӷ���ʽΪ ����ʱ��Һ��Br-��I-�����ʵ���Ũ�ȷֱ�Ϊc(Br-)�� ��c(I-)�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�������ĵ����һ�и߶�6���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��NA��ʾ�����ӵ�������ֵ�����������в��������
A��1 mol Cl2������NaOH��Ӧ��ת�Ƶĵ�����ΪNA
B�����³�ѹ�£�11.2 L�����к��е���ԭ����С��2 NA
C��1 mol̼ϩ(��CH2 )�����ĵ�����ĿΪ6NA
D��T��ʱ��1 L pH=6�Ĵ�ˮ�к�OH-��Ϊ10-6 NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ӱ�����һ�и�һ�ϵڶ����¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
����98%��Ũ���ᣨ�ѣ�1.84g/cm3�����Ƴ�Ũ��Ϊ0.5mol/L��ϡ����480mL��
��1��ѡ�õ���Ҫ���������У�
��_______����_______����_______����______����________��
��2����Ҫ�ش��������⣺����Ũ��������Ϊ_______mL������һλС������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ӱ�����һ�и�һ�ϵڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
ͬ��ͬѹ�£�A LCH4��H2�Ļ������ǡ����A LO2��ȫ��Ӧ����CO2��H2O����ԭ��������ƽ����Է�������Ϊ�� ��
A��5.56 B��6.67 C��11.12 D��9
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�����ɹŰ�ͷһ�и�һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����й���Һ��ɵ������������ǣ� ��
A����ɫ��Һ�п��ܴ�������Al3+��NH4+��Cl?��H+
B��������Һ�п��ܴ�������Na+��ClO?��SO42?��I ?
C��������Һ�п��ܴ�������Na+��K+��Cl?��HCO3?
D��������Һ�п��ܴ�������Fe3+��K+��Cl?��I ?
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com