
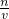 �����жϣ�
�����жϣ�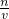 ��������ԭ����ע��̼���Ƴ���Ӧ�ڲ��������ڳ�����������ƽ�ĸ���Ϊ0.1��
��������ԭ����ע��̼���Ƴ���Ӧ�ڲ��������ڳ�����������ƽ�ĸ���Ϊ0.1�� 


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��18�֣�ijͬѧ������450mL 0.1mol/L��̼������Һ���Ը�����ѧ�ش��������⡣
��1��������5mol/L��̼������Һ������Һ����Ҫ�õ�̼������Һ�����Ϊ ��
��2������̼���ƹ������ƣ�һ�����õ���������________________________
A��450ml����ƿ B��500 ml����ƿ C����Ͳ D����ͷ�ι�
E���ձ� F�������� G��������ƽ
��3����ȷ����������Һ�����г���������ȷ���� �� ��
A����Na2CO3 4.8�� B��Na2CO35.3��
C����Na2CO3��10H2O 12.87�� D����Na2CO3��10H2O14.30��
��4����������Һ�Ĺ������ý�ͷ�ι�������ƿ�м�ˮʱ����С��Һ�泬���˿̶ȣ������ķ�����________������ţ���
A����������Һ�壬ʹ��Һ����̶�������
B��С�ļ�������ƿ����������ʹ��Һ����̶�������
C�����������һ������̼���ƹ���
D����������
��5�����в������ܵ��½��ƫ�ߵ��� ��
A��ת�ƺ�û��ϴ���ձ�
B������ʱ����
C������ʱ����
D����ȡ��Na2CO3��Һ�л�������Na2CO3��10H2O
E������Һǰ��̼������Һ��ϴ����ƿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��㶫ʡ������ѧ������ѧ�ڵ�һ���¿������ۣ���ѧ���� ���ͣ�ʵ����
��18�֣�ijͬѧ������450mL 0.1mol/L��̼������Һ���Ը�����ѧ�ش��������⡣
��1��������5mol/L��̼������Һ������Һ����Ҫ�õ�̼������Һ�����Ϊ ��
��2������̼���ƹ������ƣ�һ�����õ���������________________________
A��450ml����ƿ B��500 ml����ƿ C����Ͳ D����ͷ�ι�
E���ձ� F�������� G��������ƽ
��3����ȷ����������Һ�����г���������ȷ���� �� ��
A����Na2CO3 4.8�� B��Na2CO3 5.3��
C����Na2CO3��10H2O 12.87�� D����Na2CO3��10H2O 14.30��
��4����������Һ�Ĺ������ý�ͷ�ι�������ƿ�м�ˮʱ����С��Һ�泬���˿̶ȣ������ķ�����________������ţ���
A����������Һ�壬ʹ��Һ����̶�������
B��С�ļ�������ƿ����������ʹ��Һ����̶�������
C�����������һ������̼���ƹ���
D����������
��5�����в������ܵ��½��ƫ�ߵ��� ��
A��ת�ƺ�û��ϴ���ձ�
B������ʱ����
C������ʱ����
D����ȡ��Na2CO3��Һ�л�������Na2CO3��10H2O
E������Һǰ��̼������Һ��ϴ����ƿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��㶫ʡ������ѧ�ڵ�һ���¿������ۣ���ѧ���� ���ͣ�ʵ����
��18�֣�ijͬѧ������450mL 0.1mol/L��̼������Һ���Ը�����ѧ�ش��������⡣
��1��������5mol/L��̼������Һ������Һ����Ҫ�õ�̼������Һ�����Ϊ ��
��2������̼���ƹ������ƣ�һ�����õ���������________________________
A��450ml����ƿ B��500 ml����ƿ C����Ͳ D����ͷ�ι�
E���ձ� F�������� G��������ƽ
��3����ȷ����������Һ�����г���������ȷ���� �� ��
A����Na2CO3 4.8�� B��Na2CO3 5.3��
C����Na2CO3��10H2O 12.87�� D����Na2CO3��10H2O 14.30��
��4����������Һ�Ĺ������ý�ͷ�ι�������ƿ�м�ˮʱ����С��Һ�泬���˿̶ȣ������ķ�����________������ţ���
A����������Һ�壬ʹ��Һ����̶�������
B��С�ļ�������ƿ����������ʹ��Һ����̶�������
C�����������һ������̼���ƹ���
D����������
��5�����в������ܵ��½��ƫ�ߵ��� ��
A��ת�ƺ�û��ϴ���ձ�
B������ʱ����
C������ʱ����
D����ȡ��Na2CO3 ��Һ�л�������Na2CO3��10H2O
E������Һǰ��̼������Һ��ϴ����ƿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧ������450mL 0.1mol/L��̼������Һ���Ը�����ѧ�ش��������⡣
��1��������5mol/L��̼������Һ������Һ����Ҫ�õ�̼������Һ�����Ϊ ��
��2������̼���ƹ������ƣ�һ�����õ���������________________________
A��450ml����ƿ B��500 ml����ƿ C����Ͳ D����ͷ�ι�
E���ձ� F�������� G��������ƽ
��3����ȷ����������Һ�����г���������ȷ���� �� ��
A����Na2CO3 4.8�� B��Na2CO3 5.3��
C����Na2CO3��10H2O 12.87�� D����Na2CO3��10H2O 14.30��
��4����������Һ�Ĺ������ý�ͷ�ι�������ƿ�м�ˮʱ����С��Һ�泬���˿̶ȣ������ķ�����________������ţ���
A����������Һ�壬ʹ��Һ����̶�������
B��С�ļ�������ƿ����������ʹ��Һ����̶�������
C�����������һ������̼���ƹ���
D����������
��5�����в������ܵ��½��ƫ�ߵ��� ��
A��ת�ƺ�û��ϴ���ձ�
B������ʱ����
C������ʱ����
D����ȡ��Na2CO3 ��Һ�л�������Na2CO3��10H2O
E������Һǰ��̼������Һ��ϴ����ƿ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com