
����Ŀ������±��ش���������(��Ϊ�����µ�����)��
�� | ���볣��(Ka) |
CH3COOH | 1.8��10��5 |
HClO | 3��10��8 |
H2CO3 | K1��4.4��10��7 K2��4.7��10��11 |
H2C2O4 | K1��5.4��10��2 K2��5.4��10��5 |
H2S | K1��1.3��10��7 K2��7.1��10��15 |
��ش��������⣺
(1)ͬŨ�ȵ�CH3COO����![]() ��
��![]() ��
��![]() ��ClO����S2����H����������������_____________��
��ClO����S2����H����������������_____________��
(2)������0.1molL��1��CH3COOH��Һ�ڼ�ˮϡ�����У����б���ʽ������һ����С����_____________(����ĸ)��
A��c(H��) B��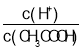 C��
C��![]() D��c(OH��)
D��c(OH��)
(3)pH��ͬ��NaClO��CH3COOK��Һ������Һ�����ʵ���Ũ�ȵĴ�С��ϵ��CH3COOK_____________NaClO������Һ�У�c(Na��)��c(ClO)_____________c(K��)��c(CH3COO��)(����������������������)��
(4)��0.1molL��1CH3COOH��Һ�еμ�NaOH��Һ��c(CH3COOH)��c(CH3COO��)��5��9����ʱ��ҺpH��_____________��
���𰸡�![]() AC �� = 5
AC �� = 5
��������
������Ӷ�Ӧ�����ĵ���ƽ�ⳣ��Խ������������������Խ����CH3COOH��Һ��ˮϡ���̣��ٽ����룬c(H+����С��c(OH-������Kw���䣻�������Խ������Ӧ��������ӵ�ˮ��̶�Խ���ж�CH3COO-��ClO-ˮ��̶ȴ�С�����õ���غ�ó�����Ũ�ȴ�С�Ĺ�ϵ����Ũ����ͬ��NaClO��CH3COOK��Һ��pH��С��ϵ�����գ�������ҺŨ�ȡ��ٽ�ˮ��������У�������ǿ��pH������CH3COOH��Һ��k=![]() ��������Һ��c(H+�������pH=-lgc(H+�����㡣
��������Һ��c(H+�������pH=-lgc(H+�����㡣
(1)ƽ�ⳣ��Խ������������������Խ�������ڵ���ƽ�ⳣ��H2C2O4��HC2O4��CH3COOH��H2CO3��H2S��HClO��HCO3��HS����ͬŨ�ȵ�CH3COO��HCO3��CO32��HC2O4��ClO��S2���H+������������ǿ��˳��Ϊ��HC2O4��CH3COO��HCO3��ClO��CO32��S2�����H+������������HC2O4��
(2)A��CH3COOH��Һ��ˮϡ���̣��ٽ����룬c(H+)��С����A��ȷ��
B��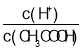 =
=![]() ��ϡ���̴ٽ�����ĵ��룬�����ӵ����ʵ�������������ʵ�����С�����Ա�ֵ���B����
��ϡ���̴ٽ�����ĵ��룬�����ӵ����ʵ�������������ʵ�����С�����Ա�ֵ���B����
C��ϡ���̣��ٽ����룬c(H+)��С��c(OH)����![]() ��С����C��ȷ��
��С����C��ȷ��
D��ϡ���̣��ٽ����룬c(H+)��С��c(OH)����D����
��ѡAC��
(3)�ݵ���ƽ�ⳣ����֪������CH3COOH��HClO����NaClO��ˮ��̶ȴ���CH3COOK��Ũ����ͬ��CH3COOK��NaClO��Һ��NaClO��ˮ��̶ȴ���CH3COOK��NaClO��Һ��pH������pH��ͬ��NaClO��CH3COOK��Һ�У�CH3COOK��Ũ�ȴ��ڴ������Һ�У����ڵ���غ㣺c(K��)+c(H��)=c(CH3COO��)+c(OH-)����c(H��)=c(CH3COO��)+c(OH-)-c(K��)����������Һ��Ҳ���ڵ���غ㣺c(Na��)+c(H��)= c(ClO-)+c(OH-)����c(H��)= c(ClO-)+c(OH-)-c(Na��)����-�ڵõ���pH��ͬ��c(H��)��ȣ�c(OH-)Ҳ��ȣ�c(Na��)��c(ClO-)=c(K��)��c(CH3COO��)��
(4)���ݴ���ĵ���ȱ���ʽCH3COOH��Һ��k=![]() =1.8��105��c(CH3COOH)��c(CH3COO)=5��9���õ���Һ��c(H+)=1.8��105��
=1.8��105��c(CH3COOH)��c(CH3COO)=5��9���õ���Һ��c(H+)=1.8��105��![]() =105mol/L������pH=5��
=105mol/L������pH=5��


 ÿ�α���ϵ�д�
ÿ�α���ϵ�д� ��ѧ����ϵ�д�
��ѧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������о������������к��еĿ�������ʹ������Ѫ�ܸ����ᡣ����������ģ����ͼ��ʾ�������йؿ��������������ȷ���ǣ�������

A.������ķ���ʽΪC9H8O4
B.�������еĺ���������ֻ���Ȼ������ǻ�
C.��������Է���������Ӧ����ȥ��Ӧ��������Ӧ���Ӿ۷�Ӧ
D.![]() �ǿ������һ��ͬ���칹�壬1mol��������������3molNaOH
�ǿ������һ��ͬ���칹�壬1mol��������������3molNaOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������HA��������NaA�Ļ����Һ���ڻ�ѧ������������Һ���������м�����������ʱ����Һ������Ա仯���ش��������⣺
(1)������Һ���ȷ֣������м�����������ʱ��������Ӧ�����ӷ���ʽΪ______����һ�ݼ�������KOH��Һʱ��������Ӧ�����ӷ���ʽΪ______��
(2)�ֽ�0.04molL-1HA��Һ��0.02molL-1NaOH��Һ�������ϣ��õ�������Һ��
����HAΪHCN���û�����Һ�Լ��ԣ�����Һ��c(Na+)______c(CN-)��(����<����=������>��)��
����HAΪCH3COOH���û�����Һ�����ԡ�����Һ�����е����Ӱ�Ũ���ɴ�С���е�˳����______��
(3)������Һһ������Ũ�Ƚϴ�����ἰ�乲�������ɣ���pHֵ�Ľ��Ƽ��㹫ʽΪ��pH=pK��+lg[c�����/c��]������ѪҺ�е�H2CO3-HCO3��ƽ���������á�ʵ��ij��ѪҺ��pH=7.2��c(![]() )=2.3��10-2mol/L������֪ѪҺ�е�H2CO3��pKa1=6.2�������ѪҺ�е�c(H2CO3)=______mol/L��
)=2.3��10-2mol/L������֪ѪҺ�е�H2CO3��pKa1=6.2�������ѪҺ�е�c(H2CO3)=______mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������D�ֳ���Ƽ���һ����Ȼ��������Ȼ�����D�Ľṹ��ͼ��ʾ��
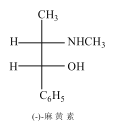
��֪D�ĺϳ�·�����£�
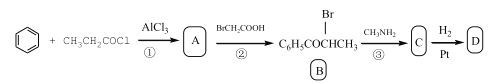
�ش��������⣺
(1)д����Ӧ�ٵĻ�ѧ����ʽ��______��ָ���䷴Ӧ����______��
(2)ָ��B�еĹ����ŵ�����______��D��������______������̼ԭ�ӡ�
(3)��C��D�Ļ�ѧ����ʽ��______��
(4)ͬʱ��������������C��ͬ���칹��(���������칹)����Ŀ��______�֡�
�����ڶ�λ��ȡ����
�ں���������(-CONH2)
(5)��֪��R����NO2![]() R����NH2�������м������ױ����������һ����
R����NH2�������м������ױ����������һ���� ���Ҵ�Ϊԭ�ϣ��Ʊ�
���Ҵ�Ϊԭ�ϣ��Ʊ� �ĺϳ�·��______(���Լ���ѡ)��
�ĺϳ�·��______(���Լ���ѡ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ҷ����������ᣬ�����ǻ�ѧѧϰС���ͬѧ�Բ��ᾧ��(H2C2O4��xH2O)���е�̽����ѧϰ�Ĺ��̣�������벢Э������������ѧϰ������ͬѧ���о������ǣ�̽���ⶨ���ᾧ��(H2C2O4��xH2O)�е�xֵ��ͨ���������Ϻ������Ѱ�ã�����������ˮ��ˮ��Һ����������KMnO4��Һ���еζ���2MnO4-��5H2C2O4��6H��===2Mn2����10CO2����8H2O,ѧϰС���ͬѧ����˵ζ��ķ����ⶨxֵ��
�ٳ�ȡ1.260 g�����ᾧ�壬�����Ƴ�100.00 mLˮ��ҺΪ����Һ��
��ȡ25.00 mL����Һ������ƿ�У��ټ���������ϡH2SO4��
����Ũ��Ϊ0.1000 mol��L��1������KMnO4����Һ���еζ����ﵽ�յ�ʱ����10.00 mL��
(1)�ζ�ʱ��������KMnO4��Һװ����ͼ�е�________(����������������)�ζ����С�
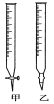
(2)��ʵ��ζ��ﵽ�յ�ı�־��___________________��
(3)ͨ���������ݣ����x��________��
���ۣ������ζ��յ�ʱ���ӵζ��̶ܿȣ����ɴ˲�õ�xֵ��________(����ƫ��������ƫС����������������ͬ)��
�����ζ�ʱ���õ�����KMnO4��Һ����ö�����Ũ�ȱ�С�����ɴ˲�õ�xֵ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ����
A.����ˮ���ȵĹ����У�Kw���pH��С
B.����FeSO4��Һʱ������ϡHNO3����Fe2��ˮ��
C.FeCl3��Һ���ɡ����������أ����յõ�FeCl3����
D.��0.1mol/L��ˮ�м�������ˮ����Һ��![]() ��С
��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���õ�ⷨ���ᴿ����ijЩ����������ʵĴ�KOH��Һ���乤��ԭ����ͼ��ʾ�������й�˵���������
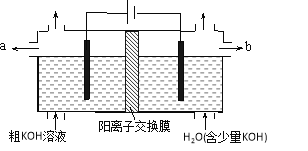
A. ͨ���������������ҺpH������
B. ������ӦʽΪ4OH��-4e����2H2O+O2��
C. ������KOH��Һ��b���ڵ���
D. K+ͨ������Ĥ������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
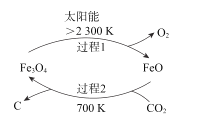
����Ŀ��������̼�Ļ��������ǻ��������о��ȵ㡣
��1����̫���ܵ������£���CO2Ϊԭ����̿�ڵ�������ͼ��ʾ���ܷ�Ӧ�Ļ�ѧ����ʽΪ__��
��2����һ����CO2�����״�ȼ�ϵķ�����CO2+3H2![]() CH3OH+H2O��
CH3OH+H2O��
��֪298K��101kPa�����£�CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(l) ��H=-akJ/mol��
CH3OH(g)+H2O(l) ��H=-akJ/mol��
2H2(g)+O2(g)=2H2O(l) ��H=-bkJ/mol��
CH3OH(g)=CH3OH(l) ��H=-ckJ/mol��
��CH3OH(l)�ı�ȼ������H=__��
��3���ڴ���M�������£�CO2�������⻯�ϳɵ�̼ϩ���ȡ�
CO2��H2ͬʱ��������������Ӧ��
��2CO2(g)+6H2(g) ![]() CH2=CH2(g)+4H2O(g) ��H<0
CH2=CH2(g)+4H2O(g) ��H<0
��2CO2(g)+6H2(g) ![]() CH3OCH3(g)+3H2O(g) ��H<0
CH3OCH3(g)+3H2O(g) ��H<0
��ͼ����ϩ����ͬʱ���ڣ���ͬ�¶��µIJ��ʣ����¶ȸ���460��ʱ��ϩ���ʽ��͵�ԭ������__��
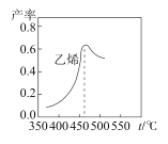
A.����M�Ļ��Խ���
B.�ٷ�Ӧ��ƽ�ⳣ�����
C.���ɼ��ѵ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������(![]() )����ǿ�����ԣ�
)����ǿ�����ԣ�![]() Эͬ����ͬʱ�����������и��ѳ��ʡ�
Эͬ����ͬʱ�����������и��ѳ��ʡ�
(1)![]() �����������������з������·�Ӧ��
�����������������з������·�Ӧ��
![]()
![]()
![]()
![]()
![]()
![]()
��Ӧ![]() ��
��![]() ________
________![]()
(2)![]() ��Ũ�ȡ�����Һ
��Ũ�ȡ�����Һ![]() ����������Ч�ʵ�Ӱ��ֱ���ͼ��ʾ��
����������Ч�ʵ�Ӱ��ֱ���ͼ��ʾ��
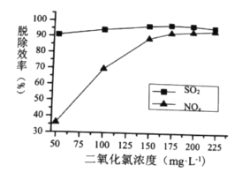
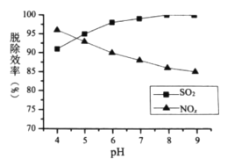
�������˵�![]() Ũ��Ϊ________
Ũ��Ϊ________![]() ��
��
��![]() ʱ������
ʱ������![]() ������
������![]() ���ѳ����½�������ܵ�ԭ����__________
���ѳ����½�������ܵ�ԭ����__________
��![]() ʱ��
ʱ��![]() �ᷢ���绯��Ӧ��
�ᷢ���绯��Ӧ��![]() ������
������![]() ��ȫ��Ӧʱ��ת�Ƶ�����ĿΪ________
��ȫ��Ӧʱ��ת�Ƶ�����ĿΪ________![]() ���绯��Ӧ���ɵ�
���绯��Ӧ���ɵ�![]() Ҳ������Ϊ����������������Ӧ��д������������
Ҳ������Ϊ����������������Ӧ��д������������![]() ��
��![]() ����Ϊ
����Ϊ![]() �����ӷ���ʽ______________��
�����ӷ���ʽ______________��
(3)��ҵ���Բ���ֲ���Ϊ���������渲�ǽ����������ʯīΪ���������![]() ��Һ�Ʊ�
��Һ�Ʊ�![]() ��д����������
��д����������![]() �ĵ缫��Ӧʽ________���˷�����ȱ�������ò�Ʒ���Ȳ��ߣ�
�ĵ缫��Ӧʽ________���˷�����ȱ�������ò�Ʒ���Ȳ��ߣ�![]() ���������������ʿ�����_____________��
���������������ʿ�����_____________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com