
ÓŠ45.45 g KClO3ŗĶMnO2µÄ»ģŗĻĪļ£¬¼ÓČČŅ»¶ĪŹ±¼äŗó£¬ÖŹĮæ±äĪŖ40.65 g£®½«“ĖŹ£Óą¹ĢĢåĘ½¾ł·Ö³ÉĮ½·Ż”£?
(1)Ņ»·Ż¼Ó×ćĮæµÄĖ®Čܽāŗ󣬹żĀĖ£¬ŌŚĀĖŅŗÖŠ¼Ó×ćĮæµÄĻõĖįĖį»ÆĮĖµÄAgNO3ČÜŅŗ£¬æÉµĆ³Įµķ¶ąÉŁæĖ£æ?
(2)ĮķŅ»·Ż¼ÓČė×ćĮæµÄÅØŃĪĖį£¬¼ÓČČŹ¹Ö®·“Ó¦£®½«·Å³öµÄĘųĢåĶØČėŗ¬KI”¢KBrµÄ1 L»ģŗĻČÜŅŗ£¬Ē”ŗĆŹ¹ČÜŅŗÖŠBr£”¢I£ĶźČ«·“Ó¦£®ČōKIµÄĪļÖŹµÄĮæÅضČÓėKBrµÄĪļÖŹµÄĮæÅضČĻąµČ£¬¶¼ĪŖ0.35 mol”¤L£1£¬ŹŌĒóKClO3µÄ·Ö½ā°Ł·ÖĀŹ”£
(1)7.175 g (2)33%?
²īĮæ·ØŹĒ»Æѧ¼ĘĖćÖŠµÄ³£ÓĆ·½·ØÖ®Ņ»”£¼õĒįµÄÖŹĮ漓ĪŖÉś³ÉµÄŃõĘųµÄÖŹĮ攣¾Ż“ĖæɼĘĖć³öÉś³ÉµÄKClÖŹĮ棬¼“Ēó³öAgCl³ĮµķµÄĮ攣?
(1)»ģŗĻĪļ¼ÓČČŗóÖŹĮæ¼õĒį45.45£40.65£½4.8 g£¬¼“Éś³ÉO2µÄÖŹĮæĪŖ4.8 g£¬»ģŗĻĪļµÄŅ»°ėæɲśÉś2.4 g O2”£?
Éč·Ö½āĮĖKClO3 x mol£¬Éś³ÉKCly mol£¬Éś³É³Įµķz g”£
2KClO3![]() 2KCl £«3O2”ü
2KCl £«3O2”ü
2 mol 2 mol 96 g?
x mol ?y mol 2.4 g
x£½y£½0£®05?
KCl£«AgNO3 = AgCl”ż£«KNO3?
1 mol 143.5 g?
0.05 mol z mol
z£½7£®175 g?
(2)ÓÉÓŚKBr”¢KIµÄĪļÖŹµÄĮæ¾łĪŖ£ŗ0.35 mol”¤L£1”Į1 L£½0.35 mol
Cl2 £« 2KBr = 2KCl£«Br2?
1 2?
n(Cl2) 0.35
n(Cl2)£½0.175 mol?
¼“ĮķŅ»°ė¹ĢĢå»ģŗĻĪļÓėÅØŃĪĖį·“Ó¦£¬¹²·Å³ö0.35 mol Cl2?
6HCl£«KClO3![]() KCl£«3Cl2”ü£«3H2O?
KCl£«3Cl2”ü£«3H2O?
122.5 g 3 mol?
m g ![]() mol
mol
4HCl£«MnO2![]() MnCl2£«Cl2”ü£«2H2O?
MnCl2£«Cl2”ü£«2H2O?
87 g 1 mol?
w g ![]() mol
mol

½āµĆ![]() ŌņKClO3µÄ·Ö½āĀŹ£½
ŌņKClO3µÄ·Ö½āĀŹ£½![]() ”Į100%£½33%£®
”Į100%£½33%£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| A | B | C | D | E | |
| n£ØCO2£© | 3 | l | 0 | 1 | l |
| n£ØH2£© | 2 | l | 0 | 1 | 2 |
| n£ØCO£© | 1 | 2 | 3 | 0.5 | 3 |
| n£ØH2O£© | 5 | 2 | 3 | 2 | l |
| T/”ćC | T1 | 300 | T2 |
| K | 1.00”Į107 | 2.45”Į105 | 1.88”Į103 |
| 1 |
| 2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗĖÄ“ØŹ”ÅŹÖ¦»ØŹŠ2012£2013ѧğøßŅ»ĻĀѧʌʌĩµ÷ŃŠ¼ą²ā»ÆѧŹŌĢā ĢāŠĶ£ŗ022
(1)ŌŚ200”ę”¢101 kPaŹ±£¬1 g””H2ÓėµāÕōĘųĶźČ«·“Ó¦·Å³ö7.45 kJµÄČČĮ棬ĒėŠ“³öøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½________£®
(2)ŌŚ1”Į105 PaŗĶ298 KŹ±£¬½«²šæŖ1 mol¹²¼Ū¼üĖłŠčŅŖµÄÄÜĮæ³ĘĪŖ¼üÄÜ(kJ/mol)£®ĻĀĆęŹĒŅ»Š©¹²¼Ū¼üµÄ¼üÄÜ£ŗ
ŌŚ298 KŹ±£¬ŌŚ“߻ƼĮ“ęŌŚĻĀ£¬H2(g)£«Cl2(g)£½2HCl(g)””¦¤H£½________kJ/mol
(3)ŅŃÖŖ£ŗCO(g)£«2H2(g)![]() CH3OH(g)””¦¤H1£½£116 kJ”¤mol£1
CH3OH(g)””¦¤H1£½£116 kJ”¤mol£1
CO(g)£«![]() O2(g)£½CO2(g)””¦¤H2£½£283 kJ”¤mol£1
O2(g)£½CO2(g)””¦¤H2£½£283 kJ”¤mol£1
H2(g)£«![]() O2(g)£½H2O(g)””¦¤H3£½£242 kJ”¤mol£1
O2(g)£½H2O(g)””¦¤H3£½£242 kJ”¤mol£1
Ōņ±ķŹ¾1 molĘųĢ¬¼×“¼ĶźČ«Č¼ÉÕÉś³ÉCO2ŗĶĖ®ÕōĘųŹ±µÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗ
________£®
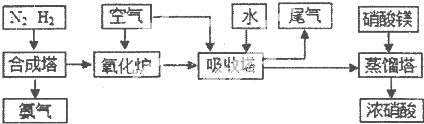
(4)N2(g)£«3H2(g)![]() 2NH3(g)·“Ó¦¹ż³ĢµÄÄÜĮæ±ä»ÆČēĶ¼ĖłŹ¾£®ŅŃÖŖ2 mol””N2(g)·“Ӧɜ³É4 mol””NH3(g)·Å³ö£188.4 kJ/mol£®Ōņ£ŗ
2NH3(g)·“Ó¦¹ż³ĢµÄÄÜĮæ±ä»ÆČēĶ¼ĖłŹ¾£®ŅŃÖŖ2 mol””N2(g)·“Ӧɜ³É4 mol””NH3(g)·Å³ö£188.4 kJ/mol£®Ōņ£ŗ

Ķ¼ÖŠEµÄ“󊔶ŌøĆ·“Ó¦µÄ·“Ó¦ČČÓŠĪŽÓ°Ļģ£æ________£®(Ģī”°ÓŠÓ°Ļģ”±»ņ”°ĪŽÓ°Ļģ”±)£®øĆ·“Ó¦Ķس£ÓĆĢś“„Ć½×÷“߻ƼĮ£¬¼ÓĢś“„Ć½»įŹ¹Ķ¼ÖŠBµćÉżøß»¹ŹĒ½µµĶ£æ________(Ģī”°Éżøß”±»ņ”°½µµĶ”±)£®Ķ¼ÖŠ¦¤H£½________kJ/mol£®¶ŌÓŚ“ļĘ½ŗāŗóµÄøĆ·“Ó¦£¬ČōÖ»øıäĻĀĮŠĢõ¼žÖ®Ņ»£¬ÄÜŹ¹µ„Ī»Ģå»żÄŚ»ī»Æ·Ö×Ó°Ł·ÖŹżŌö¼ÓµÄŹĒ________£®
A£®ÉżĪĀ
B£®ŌŁ³äČėN2ŗĶH2£¬²¢Ź¹ĖüĆĒµÄÅØ¶Č¶¼Ōö¼ÓŅ»±¶
C£®¼õŃ¹
D£®¼Ó“߻ƼĮ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğ±±¾©ŹŠĶØÖŻĒųøßȿğ¼¶ÉĻѧʌʌĩƞµ×æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
A£®2 mol/L KClČÜŅŗÓė1 mol/L K2SO4ČÜŅŗ»ģŗĻŗó£¬c(K£«)ĪŖ2 mol/L
B£®120 g NaClČÜŅŗÖŠČÜÓŠ20 g NaCl£¬øĆĪĀ¶ČĻĀNaClµÄČܽā¶ČĪŖ20g
C£®22.4 L HClĘųĢåČÜÓŚĖ®ÖĘ³É1 LČÜŅŗ£¬øĆČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ1 mo1/L
D£®°Ń5 gµØ·Æ(CuSO4∙5H2O)ČÜÓŚ45 gĖ®ÖŠ£¬ĖłµĆČÜŅŗČÜÖŹµÄÖŹĮæ·ÖŹżĪŖ10%
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğøßČżÉĻѧʌµ„ŌŖ²āŹŌ£Ø6£©»ÆѧŹŌ¾ķ£ØŠĀČĖ½Ģ°ę£© ĢāŠĶ£ŗŹµŃéĢā
£Ø10·Ö£©ĪŅ¹ś»Æ¹¤×ؼŅŗīµĀ°ńµÄ”°ŗīŹĻÖĘ¼ī·Ø”±ŌųĪŖŹĄ½ēÖĘ¼ī¹¤Ņµ×ö³öĮĖĶ»³ö¹±Ļ×”£ĖūĄūÓĆNaHCO3”¢NaCl”¢NH4C1µČĪļÖŹČܽā¶ČµÄ²īŅģ£¬ŅŌŹ³ŃĪ”¢°±Ęų”¢¶žŃõ»ÆĢ¼µČĪŖŌĮĻÖʵĆNaHCO3£¬½ų¶ųÉś²ś³ö“æ¼ī”£ŅŌĻĀA”¢B”¢C”¢DĖÄøö×°ÖĆæÉ×é×°³ÉŹµŃéŹŅÄ£Äā ”°ŗīŹĻÖĘ¼ī·Ø”±ÖĘČ”NaHCO3µÄŹµŃé×°ÖĆ”£×°ÖĆÖŠ·Ö±šŹ¢ÓŠŅŌĻĀŹŌ¼Į£ŗB£ŗĻ”ĮņĖį£»C£ŗŃĪĖį”¢Ģ¼ĖįøĘ£»D£ŗŗ¬°±µÄ±„ŗĶŹ³ŃĪĖ®”¢Ė®
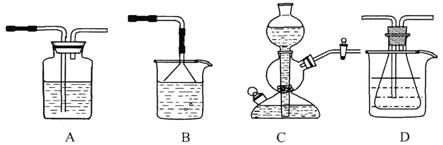
ĖÄÖÖŃĪŌŚ²»Ķ¬ĪĀ¶ČĻĀµÄČܽā¶Č£Øg£Æ100gĖ®£©±ķ
|
|
0”ę |
10”ę |
20”ę |
30”ę |
40”ę |
50”ę |
60”ę |
100”ę |
|
NaCl |
35£®7 |
35£®8 |
36£®0 |
36_3 |
36£®6 |
37£®0 |
37£®3 |
39£®8 |
|
NH4HCO3 |
11£®9 |
15£®8 |
21£®0 |
27£®0 |
”Ŗ¢Ł |
”Ŗ |
”Ŗ |
”Ŗ |
|
NaHCO3 |
6£®9 |
8£®1 |
9£®6 |
11£®1 |
12£®7 |
14£®5 |
16£®4 |
|
|
NH4Cl |
29£®4 |
33£®3 |
37£®2[Ą“Ō“:Z|xx|k.Com] |
41£®4 |
45£®8[Ą“Ō“:Z*xx*k.Com] |
50£®4 |
55£®3 |
77£®3 |
£ØĖµĆ÷£ŗ¢Ł>35”ęNH4HCO3»įÓŠ·Ö½ā£©
Ēė»Ų“šŅŌĻĀĪŹĢā£ŗ
£Ø1£©×°ÖƵÄĮ¬½ÓĖ³ŠņÓ¦ŹĒ £ØĢī×ÖÄø£©”£
£Ø2£©A×°ÖĆÖŠŹ¢·ÅµÄŹŌ¼ĮŹĒ £¬Ęä×÷ÓĆŹĒ ”£
£Ø3£©ŌŚŹµŃé¹ż³ĢÖŠ£¬ŠčŅŖæŲÖĘDĪĀ¶ČŌŚ30”ꔫ35”ę£¬ŌŅņŹĒ ”£
£Ø4£©·“Ó¦½įŹųŗ󣬽«×¶ŠĪĘ潞ŌŚĄäĖ®ÖŠ£¬Īö³öNaHCO3¾§Ģ唣ÓĆÕōĮóĖ®Ļ“µÓNaHCO3¾§ĢåµÄÄæµÄŹĒ³żČ„ ŌÓÖŹ£ØŅŌ»ÆѧŹ½±ķŹ¾£©
£Ø5£©½«×¶ŠĪĘæÖŠµÄ²śĪļ¹żĀĖŗó£¬ĖłµĆµÄÄøŅŗÖŠŗ¬ÓŠ £ØŅŌ»ÆѧŹ½±ķŹ¾£©£¬¼ÓČėĀČ»ÆĒā£¬²¢½ųŠŠ ²Ł×÷£¬Ź¹NaClČÜŅŗŃ»·Ź¹ÓĆ£¬Ķ¬Ź±æÉ»ŲŹÕNH4C1”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com