
ĻĀĮŠŹµŃ铦ĄķæÉŠŠµÄŹĒ£Ø £©
¢Ł£®½«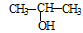 ÓėNaOHµÄ“¼ČÜŅŗ¹²ČČÖʱøCH3”ŖCHØTCH2
ÓėNaOHµÄ“¼ČÜŅŗ¹²ČČÖʱøCH3”ŖCHØTCH2
¢Ś£®Ļņ¼×ĖįŗĶ¼×Č©µÄ»ģŗĻĪļÖŠ¼ÓČėĒāŃõ»ÆÄĘČÜŅŗ£¬ÖŠŗĶ¼×Ėįŗ󣬼ÓČėŠĀÖʵÄĒāŃõ»ÆĶ¼ÓČČ”Ŗ”Ŗ¼ģŃé»ģŗĻĪļÖŠŹĒ·ńŗ¬ÓŠ¼×Č©
¢Ū£®Ļņ±ūĻ©Č©£ØCH2=CH”ŖCHO£©ÖŠµĪČėKMnO4(H+)ČÜŅŗ£¬¹Ū²ģ×ĻÉ«ĶŹČ„£¬ÄÜÖ¤Ć÷½į¹¹ÖŠ“ęŌŚĢ¼Ģ¼Ė«¼ü
¢Ü£®ŹµŃ鏱ŹÖÖø²»Š”ŠÄÕ“ÉĻ±½·Ó£¬Į¢¼“ÓĆ70oŅŌÉĻµÄČČĖ®ĒåĻ“
A£®Ö»ÓŠ¢Ł B£®Ö»ÓŠ¢Ł¢Ü C£®Ö»ÓŠ¢Ł¢Ū¢Ü D£®¶¼²»ÄÜ
 ѧĮ·æģ³µµĄæŚĖćŠÄĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø
ѧĮ·æģ³µµĄæŚĖćŠÄĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
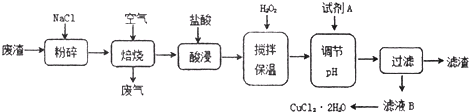
| ĒāŃõ»ÆĪļæŖŹ¼³ĮµķŹ±µÄpH | ĒāŃõ»ÆĪļ³ĮµķĶźČ«Ź±µÄpH | |
| Fe3+ | 1.9 | 3.2 |
| Fe2+ | 7.0 | 9.0 |
| Cu2+ | 4.7 | 6.7 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗŗÓ±±Ź”ŗāĖ®ÖŠŃ§2011£2012ѧğø߶žÉĻѧʌĖĵ÷æ¼ŹŌ»ÆѧŹŌĢā ĢāŠĶ£ŗ021
|
ĻĀĮŠŹµŃ铦ĄķæÉŠŠµÄŹĒ ¢Ł½« ¢ŚĻņ¼×ĖįŗĶ¼×Č©µÄ»ģŗĻĪļÖŠ¼ÓČėĒāŃõ»ÆÄĘČÜŅŗ£¬ÖŠŗĶ¼×Ėįŗ󣬼ÓČėŠĀÖʵÄĒāŃõ»ÆĶ¼ÓČČ”Ŗ”Ŗ¼ģŃé»ģŗĻĪļÖŠŹĒ·ńŗ¬ÓŠ¼×Č© ¢ŪĻņ±ūĻ©Č© (CH2£½CH£CHO)ÖŠµĪČėKMnO4(H+)ČÜŅŗ£¬¹Ū²ģ×ĻÉ«ĶŹČ„£¬ÄÜÖ¤Ć÷½į¹¹ÖŠ“ęŌŚĢ¼Ģ¼Ė«¼ü¢ÜŹµŃ鏱ŹÖÖø²»Š”ŠÄÕ“ÉĻ±½·Ó£¬Į¢¼“ÓĆ70”ćŅŌÉĻµÄČČĖ®ĒåĻ“ | |
A£® |
Ö»ÓŠ¢Ł |
B£® |
Ö»ÓŠ¢Ł¢Ü |
C£® |
Ö»ÓŠ¢Ł¢Ū¢Ü |
D£® |
¶¼²»ÄÜ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŹµŃ铦ĄķæÉŠŠµÄŹĒ£Ø £©
¢Ł£®½«ÓėNaOHµÄ“¼ČÜŅŗ¹²ČČÖʱøCH3”ŖCHØTCH2
¢Ś£®Ļņ¼×ĖįŗĶ¼×Č©µÄ»ģŗĻĪļÖŠ¼ÓČėĒāŃõ»ÆÄĘČÜŅŗ£¬ÖŠŗĶ¼×Ėįŗ󣬼ÓČėŠĀÖʵÄĒāŃõ»ÆĶ¼ÓČČ”Ŗ”Ŗ¼ģŃé»ģŗĻĪļÖŠŹĒ·ńŗ¬ÓŠ¼×Č©
¢Ū£®Ļņ±ūĻ©Č©£ØCH2=CH”ŖCHO£©ÖŠµĪČėKMnO4(H+)ČÜŅŗ£¬¹Ū²ģ×ĻÉ«ĶŹČ„£¬ÄÜÖ¤Ć÷½į¹¹ÖŠ“ęŌŚĢ¼Ģ¼Ė«¼ü
¢Ü£®ŹµŃ鏱ŹÖÖø²»Š”ŠÄÕ“ÉĻ±½·Ó£¬Į¢¼“ÓĆ70oŅŌÉĻµÄČČĖ®ĒåĻ“
A£®Ö»ÓŠ¢Ł B£®Ö»ÓŠ¢Ł¢Ü C£®Ö»ÓŠ¢Ł¢Ū¢Ü D£®¶¼²»ÄÜ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğŗÓ±±Ź”ŗāĖ®ÖŠŃ§ø߶žÉĻѧʌĖĵ÷æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠŹµŃ铦ĄķæÉŠŠµÄŹĒ£Ø £©
¢Ł£®½«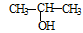 ÓėNaOHµÄ“¼ČÜŅŗ¹²ČČÖʱøCH3”ŖCHØTCH2
ÓėNaOHµÄ“¼ČÜŅŗ¹²ČČÖʱøCH3”ŖCHØTCH2
¢Ś£®Ļņ¼×ĖįŗĶ¼×Č©µÄ»ģŗĻĪļÖŠ¼ÓČėĒāŃõ»ÆÄĘČÜŅŗ£¬ÖŠŗĶ¼×Ėįŗ󣬼ÓČėŠĀÖʵÄĒāŃõ»ÆĶ¼ÓČČ”Ŗ”Ŗ¼ģŃé»ģŗĻĪļÖŠŹĒ·ńŗ¬ÓŠ¼×Č©
¢Ū£®Ļņ±ūĻ©Č©£ØCH2=CH”ŖCHO£©ÖŠµĪČėKMnO4(H+)ČÜŅŗ£¬¹Ū²ģ×ĻÉ«ĶŹČ„£¬ÄÜÖ¤Ć÷½į¹¹ÖŠ“ęŌŚĢ¼Ģ¼Ė«¼ü
¢Ü£®ŹµŃ鏱ŹÖÖø²»Š”ŠÄÕ“ÉĻ±½·Ó£¬Į¢¼“ÓĆ70oŅŌÉĻµÄČČĖ®ĒåĻ“
| A£®Ö»ÓŠ¢Ł | B£®Ö»ÓŠ¢Ł¢Ü | C£®Ö»ÓŠ¢Ł¢Ū¢Ü | D£®¶¼²»ÄÜ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com