

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)CuCl�Ʊ���������Ҫ������������Ϊ20.0%��CuSO4��Һ���Լ������Ƹ���Һ�����CuSO4��5H2O��H2O������֮�ȡ�
(2)ȷ��ȡ���Ʊ���0.250 0 g CuCl��Ʒ����һ������0.5 mol��L-1 FeCl3��Һ�У�����Ʒ��ȫ�ܽ��ˮ20 mL����0.100 0 mol��L-1��Ce(SO4)2��Һ�ζ����յ㣬����24.60 mL Ce (SO4)2��Һ���йػ�ѧ��ӦΪ��
Fe3+CuCl====Fe2++Cu2++Cl-
Ce4++Fe2+====Fe3++Ce3+
ͨ������˵��������Ʒ��CuCl�����������Ƿ���ϱ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�����ʡ��У�����ڶ����������ۻ�ѧ�Ծ��������棩 ���ͣ������
�Ȼ���ͭ(CuCl)�������л��ϳɹ�ҵ�еĴ�������һ�ְ�ɫ��ĩ������ˮ���������Ҵ���ϡ����ڿ�����Ѹ�ٱ���������ɫ��������ֽ⣬��ɺ�ɫ����ͼ�ǹ�ҵ��������ӡˢ��·�ķ�Һ����Fe3+��Cu2+��Fe2+��Cl-������CuCl���������£�


����������Ϣ�ش��������⣺
��1�����������̻��������ȼҵ�����Ṥҵ�������ϣ���ҵ��������ķ�����______________���ȼҵ��װ����_____________________��
��2��д������������X__________? Y___________ ���ѧʽ��
��3��д������CuCl�Ļ�ѧ����ʽ________________________________________________________��
��4��������Ϊ�����CuCl��Ʒ������������______________�����ٹ��ˣ�������CuCl���岻��ˮ������ˮ�Ҵ�ϴ�ӵ�Ŀ����______________________________�����������е�����Һ��pH���ܹ����ԭ����______________________________��
��5����CuCl�����ɹ����������ϲ���Ҫ����SO2���壬��������__________________________��
��6����CuCl�����ɹ����г��������⡢��ȫ�����⣬����Ϊ��Ӧ��ע��Ĺؼ�������:
_____________________________________��
��7���Ȼ���ͭ�Ķ���������
����ȡ��Ʒ0.25g(����0.0002g)����Ԥ�ȷ��벣����50����10ml������FeCl3��Һ250ml��ƿ�У�����ҡ�����������������____________________________��
������Ʒ�ܽ��ˮ50ml���ڷ�����ָʾ��2�Σ�
��������0.10 mol��L-1���������Һ������ɫ����Ϊ�յ㣻ͬʱ���հ�����һ�Ρ���֪��CuCl + FeCl3 =CuCl2 + FeCl2????? Fe2+ + Ce4+ = Fe3+ + Ce3+
������ظ����β�ã�
| 1 | 2 | 3 |
�հ�ʵ���������������Һ�����(ml) | 0.75 | 0.50 | 0.80 |
0.25����Ʒ�������������Һ�����(ml) | 24.65 | 24.75 | 24.70 |
�����ݴ����������CuCl�Ĵ���Ϊ____________����ƽ��ʵ�������ܳ���0.3%��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�����ʡ�����и������Ĵ��¿���ѧ�Ծ��������棩 ���ͣ������
�Ȼ���ͭ(CuCl)�ǰ�ɫ��ĩ��������ˮ���Ҵ����۵�422 �棬�е�1366 �棬�ڿ�����Ѹ�ٱ���������ɫ���������л��ϳɹ�ҵ�еĴ������Դ���ˮ(��Ca2����Mg2����SO42��������)��Cu��ϡ���ᡢSO2��Ϊԭ�Ϻϳ�CuCl�Ĺ������£�

��1���ڴ��γ��ӵķ�ӦI�м�Na2CO3��Һ�������� ��
��������Ҫ�ɷ֣� ��
��2����Ӧ����ɺ���Һ����Ҫ������ ��
��3����ӦIV��VI�����Ʊ�CuCl�Ļ�ѧ���̣�
�ٷ�ӦIV�����Cu�����������Ŀ���� ��
��д����ӦVI�����ӷ���ʽ ��
��4����ӦVI���˵õ�CuCl����������ˮ�Ҵ�ϴ�ӳ���������ո��������70 �����2Сʱ����ȴ���ܷ��װ���ò�Ʒ����70����ո����Ŀ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�����ʡ�����и������Ĵ��¿���ѧ�Ծ��������棩 ���ͣ������
�Ȼ���ͭ(CuCl)�ǰ�ɫ��ĩ��������ˮ���Ҵ����۵�422 �棬�е�1366 �棬�ڿ�����Ѹ�ٱ���������ɫ���������л��ϳɹ�ҵ�еĴ������Դ���ˮ(��Ca2����Mg2����SO42��������)��Cu��ϡ���ᡢSO2��Ϊԭ�Ϻϳ�CuCl�Ĺ������£�

��1���ڴ��γ��ӵķ�ӦI�м�Na2CO3��Һ�������� ��
��������Ҫ�ɷ֣� ��
��2����Ӧ����ɺ���Һ����Ҫ������ ��
��3����ӦIV��VI�����Ʊ�CuCl�Ļ�ѧ���̣�
�ٷ�ӦIV�����Cu�����������Ŀ���� ��
��д����ӦVI�����ӷ���ʽ ��
��4����ӦVI���˵õ�CuCl����������ˮ�Ҵ�ϴ�ӳ���������ո��������70 �����2Сʱ����ȴ���ܷ��װ���ò�Ʒ����70����ո����Ŀ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�����ʡ�����и������Ĵ��¿���ѧ�Ծ��������棩 ���ͣ������
�Ȼ���ͭ(CuCl)�ǰ�ɫ��ĩ��������ˮ���Ҵ����۵�422 �棬�е�1366 �棬�ڿ�����Ѹ�ٱ���������ɫ���������л��ϳɹ�ҵ�еĴ������Դ���ˮ(��Ca2����Mg2����SO42��������)��Cu��ϡ���ᡢSO2��Ϊԭ�Ϻϳ�CuCl�Ĺ������£�
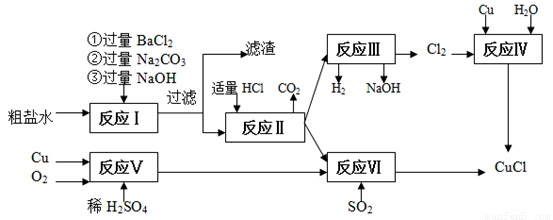
��1���ڴ��γ��ӵķ�ӦI�м�Na2CO3��Һ�������� ��
��������Ҫ�ɷ֣� ��
��2����Ӧ����ɺ���Һ����Ҫ������ ��
��3����ӦIV��VI�����Ʊ�CuCl�Ļ�ѧ���̣�
�ٷ�ӦIV�����Cu�����������Ŀ���� ��
��д����ӦVI�����ӷ���ʽ ��
��4����ӦVI���˵õ�CuCl����������ˮ�Ҵ�ϴ�ӳ���������ո��������70 �����2Сʱ����ȴ���ܷ��װ���ò�Ʒ����70����ո����Ŀ���� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com