
| |||||||||||||||||||||||||||||||
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ����ѧ--ѡ��3�����ʽṹ�����ʡ�
����ѧ--ѡ��3�����ʽṹ�����ʡ�
| ||
| 2 |
| ||
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
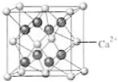 ��4��B��Ca�γɵľ���ľ�������ͼ��ʾ������Ca2+����λ����
��4��B��Ca�γɵľ���ľ�������ͼ��ʾ������Ca2+����λ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ۻ�ѧ����ѡ��ѧ�뼼���ݣ�15�֣�
�ǻ���ʯ[Ca5��PO4��3OH]��һ��һ����Ҫ�����������ϡ��䳣�õ��Ʊ����������֣�
����A����Ũ��ˮ�ֱ��Ca��NO3��2�ͣ�NH4��2HPO4��Һ��pHԼΪ12���ھ��ҽ����£�����NH4��2HPO4��Һ��������Ca��NO3��2��Һ�С�
����B�����ҽ����£���H3PO4��Һ�����μӵ�Ca��OH��2����Һ�С�
3�ָ��ε��ܽ������ҺpH�ı仯����ͼ��ʾ��ͼ���������Ǹ�����Ũ�ȵĶ��������ش��������⣺
��1����ɷ���A�ͷ���B���Ʊ�Ca5��PO4��3OH�Ļ�ѧ��Ӧ����ʽ��
��5 Ca��NO3��2 + 3��NH4��2HPO4 + 4NH3��H2O = Ca5��PO4��3OH��+ + ��2�֣�
��5Ca��OH��2 + 3H3PO4 = ��2�֣�
��2���뷽��A��ȣ�����B���ŵ��� ����3�֣�
��3������B�У����H3PO4��Һ�μӹ��죬�ƵõIJ��ﲻ������ԭ����
����3�֣�
��4��ͼ����ʾ3�ָ��������������ȶ��Ĵ�����ʽ�� ���ѧʽ������2�֣�
��5����մ���������ϣ���ø�������²����������ʣ������ȣ�ݡ���ϻ�ѧƽ���ƶ�ԭ����������ԭ�� ����3�֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ĸ�����ѧ�ڵڶ����¿������ۣ���ѧ���� ���ͣ������
�ۻ�ѧ����ѡ��ѧ�뼼���ݣ�15�֣�
�ǻ���ʯ[Ca5��PO4��3OH]��һ��һ����Ҫ�����������ϡ��䳣�õ��Ʊ����������֣�
����A����Ũ��ˮ�ֱ��Ca��NO3��2�ͣ�NH4��2HPO4��Һ��pHԼΪ12���ھ��ҽ����£�����NH4��2HPO4��Һ��������Ca��NO3��2��Һ�С�
����B�����ҽ����£���H3PO4��Һ�����μӵ�Ca��OH��2����Һ�С�

3�ָ��ε��ܽ������ҺpH�ı仯����ͼ��ʾ��ͼ���������Ǹ�����Ũ�ȵĶ��������ش��������⣺
��1����ɷ���A�ͷ���B���Ʊ�Ca5��PO4��3OH�Ļ�ѧ��Ӧ����ʽ��
��5 Ca��NO3��2 + 3��NH4��2HPO4 + 4NH3��H2O = Ca5��PO4��3OH��+ + ��2�֣�
��5Ca��OH��2 + 3H3PO4 = ��2�֣�
��2���뷽��A��ȣ�����B���ŵ��� ����3�֣�
��3������B�У����H3PO4��Һ�μӹ��죬�ƵõIJ��ﲻ������ԭ����
����3�֣�
��4��ͼ����ʾ3�ָ��������������ȶ��Ĵ�����ʽ�� ���ѧʽ������2�֣�
��5����մ���������ϣ���ø�������²����������ʣ������ȣ�ݡ���ϻ�ѧƽ���ƶ�ԭ����������ԭ�� ����3�֣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com