
ΓΨΧβΡΩΓΩ¥”“Μ÷÷Κ§“χΩσ(Κ§AgΓΔZnΓΔCuΓΔPbΦΑ…ΌΝΩSiO2)÷–Χα»ΓAgΓΔCuΦΑPbΒΡΙΛ“’Νς≥Χ»γœ¬:
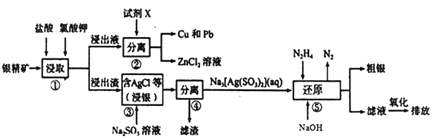
(1)≤Ϋ÷ηΔΌΧαΗΏΩσΈο÷–Ϋπ τάκΉ”Ϋΰ»Γ¬ Θ§≥ΐΩ…ΗΡ±δ―ΈΥαΒΡ≈®Ε»ΚΆ¬»ΥαΦΊΒΡΝΩΆβΘ§ΜΙΩ…≤…»ΓΒΡ¥κ © «__________(–¥≥ωΝΫ÷÷Φ¥Ω…)
(2)≤Ϋ÷ηΔΎ÷– ‘ΦΝXΈΣ______ (ΧνΜ·―ß ΫΘ§œ¬Ά§)ΘΜ≤Ϋ÷ηΔή¬Υ‘ϋΒΡ≥…Ζ÷ΈΣ_______ΓΘ
(3)≤Ϋ÷ηΔέΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ____________________ΓΘ
(4)≤Ϋ÷ηΔίΖΔ…ζΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ____________________ΘΜΤδ÷–N2H4(κ¬) ΒΡΒγΉ” ΫΈΣ____ΓΘ
(5)ΓΑΜΙ‘≠Γ±ΚσΒΡΓΑ¬Υ“ΚΓ±Ψ≠―θΜ·ΚσΘ§Τδ÷–ΒΡ»ή÷ ÷ς“ΣΈΣ_______ΓΘ
(6) “―÷Σ≥ΘΈ¬œ¬Θ§Ksp(AgCl)=1.8ΓΝ10-10Θ§Ksp(AgI)=1.0ΓΝ10-16ΓΘ»τ‘ΎAgC1ΒΡ–ϋΉ«“Κ÷–Φ”»κNaCl ΙΧΧεΘ§AgCl ≥ΝΒμΒΡ»ήΫβΕ»_____ (ΧνΓΑ…ΐΗΏΓ±ΓΔΓΑ≤Μ±δΓ±ΜρΓΑΫΒΒΆΓ±)Θ§ΆυAgCl ΒΡ–ϋΉ«“Κ÷–ΒΈΦ”NaI»ή“ΚΘ§Β±AgCl ΩΣ ΦΉΣΜ·ΈΣAgI ±Θ§I-ΒΡ≈®Ε»±Ί–κ≤ΜΒΆ”Ύ_____mol/L (≤Μ”ΟΜ·Φρ)ΓΘ
ΓΨ¥πΑΗΓΩ Β±ΧαΗΏΫΰ»ΓΈ¬Ε»ΓΔ Β±―”≥ΛΫΰ»Γ ±ΦδΓΔΫΝΑηΓΔΫΪΩσΈοΖέΥι Zn SiO2 AgCl+2Na2SO3==Na3[Ag(SO3)2]+NaCl 4[Ag(SO3)2]3-+N2H4+4OH-=4AgΓΐ+8SO32-+N2Γϋ+4H2O 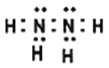 Na2SO4 ΫΒΒΆ
Na2SO4 ΫΒΒΆ ![]()
ΓΨΫβΈωΓΩ“χΨΪΩσ(ΤδΜ·―ß≥…Ζ÷”–ΘΚAgΓΔZnΓΔCuΓΔPbΦΑ…ΌΝΩSiO2Β»)Φ”―ΈΥαΚΆ¬»ΥαΦΊΫΰ»ΓΘ§Ιΐ¬ΥΘ§»ή“Κ÷–Κ§”–Zn2+ΓΔCu2+ΓΔPb2+Θ§¬Υ‘ϋ÷–Κ§”–SiO2ΓΔAgClΒ»ΘΜΫΰ≥ω“Κ÷–Φ”Ϋπ τΜΙ‘≠ΦΝZnΘ§Α―Cu2+ΓΔPb2+ΜΙ‘≠ΈΣΒΞ÷ Θ§‘ρ ‘ΦΝXΈΣZnΘ§Ζ÷άκ≤ΌΉςΔΎΈΣΙΐ¬ΥΘ§ΒΟΒΫCuΚΆPbΓΔZnCl2»ή“ΚΘΜΫΰ≥ω‘ϋΚ§”–SiO2ΓΔAgClΘ§Φ”Na2SO3»ή“ΚΘ§AgCl”κNa2SO3Ζ¥”Π…ζ≥…Na3[Ag(SO3)2]ΚΆNaClΘ§Ιΐ¬ΥΘ§¬Υ‘ϋΈΣSiO2Θ§¬Υ“ΚΈΣNa3[Ag(SO3)2]ΚΆNaClΘ§‘Ύ¬Υ“Κ÷–Φ”N2H4ΚΆ«β―θΜ·ΡΤΘ§…ζ≥…AgΚΆΒΣΤχΘΜ¬Υ“Κ÷–Κ§”–―«ΝρΥαΡΤΘ§―θΜ·…ζ≥…ΝρΥαΡΤΚσ≈≈Ζ≈ΓΘ
(1)ΈΣΧαΗΏΫΰ»Γ¬ Ω…≤…»ΓΒΡ¥κ ©Θ§≥ΐΩ…ΗΡ±δ―ΈΥαΒΡ≈®Ε»ΚΆ¬»ΥαΦΊΒΡΝΩΆβΘ§ΜΙΩ…“‘ Β±Χα»ΓΫΰ»ΓΈ¬Ε»Μρ Β±―”≥ΛΫΰ»Γ ±ΦδΜρ≥δΖ÷ΫΝΑηΒ»Θ§Ι ¥πΑΗΈΣΘΚ Β±Χα»ΓΫΰ»ΓΈ¬Ε»Μρ Β±―”≥ΛΫΰ»Γ ±ΦδΜρ≥δΖ÷ΫΝΑηΘΜ
(2)”…Νς≥ΧΖ÷ΈωΩ…÷ΣΘ§ ‘ΦΝXΈΣZnΘ§≤Ϋ÷ηΔή¬Υ‘ϋ÷–≥ΐΚ§ΒΞ÷ ΝρΆβΘ§ΜΙΚ§”–ΒΡ≥…Ζ÷”–SiO2ΘΜ
Ι ¥πΑΗΈΣΘΚZnΘΜSiO2ΘΜ
(3)≤Ϋ÷ηΔέΦ”Na2SO3»ή“ΚΘ§AgCl”κNa2SO3Ζ¥”Π…ζ≥…Na3[Ag(SO3)2]ΚΆNaClΘ§Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣAgCl+2Na2SO3=Na3[Ag(SO3)2]+NaClΘ§Ι ¥πΑΗΈΣΘΚAgCl+2Na2SO3=Na3[Ag(SO3)2]+NaClΘΜ
(4)¬Υ“ΚΈΣNa3[Ag(SO3)2]ΚΆNaClΘ§‘Ύ¬Υ“Κ÷–Φ”N2H4Θ§Na3[Ag(SO3)2]”κN2H4Ζ¥”Π…ζ≥…AgΚΆΒΣΤχΓΔ―«ΝρΥαΡΤΘ§Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣΘΚ[Ag(SO3)2]3-+N2H4+4OH-=4AgΓΐ+8SO32-+N2Γϋ+4H2OΘ§Τδ÷–N2H4(κ¬)ΒΡΒγΉ” ΫΈΣ![]() Θ§Ι ¥πΑΗΈΣΘΚ[Ag(SO3)2]3-+N2H4+4OH-=4AgΓΐ+8SO32-+N2Γϋ+4H2OΘΜ
Θ§Ι ¥πΑΗΈΣΘΚ[Ag(SO3)2]3-+N2H4+4OH-=4AgΓΐ+8SO32-+N2Γϋ+4H2OΘΜ![]() ΘΜ
ΘΜ
(5)¬Υ“Κ÷–Κ§”–―«ΝρΥαΡΤΘ§―θΜ·…ζ≥…ΝρΥαΡΤΘΜ‘ρΓΑΜΙ‘≠Κσ“ΚΓ±Ψ≠―θΜ·ΚσΘ§≈≈Ζ≈“Κ÷–»ή÷ ΒΡ÷ς“Σ≥…Ζ÷ΈΣNa2SO4Θ§Ι ¥πΑΗΈΣΘΚNa2SO4ΘΜ
(6)‘ΎAgC1ΒΡ–ϋΉ«“Κ÷–Φ”»κNaClΙΧΧεΘ§¬»άκΉ”≈®Ε»‘ω¥σΘ§ ΙAgClΒΡ»ήΫβΤΫΚβΡφœρ“ΤΕ·Θ§AgCl≥ΝΒμΒΡ»ήΫβΕ»ΫΒΒΆΘΜ‘ΎAgClΒΡ–ϋΉ«“Κ÷–c(Cl-)= c(Ag+)=![]() =
=![]() mol/LΘ§ΒΈΦ”NaI»ή“ΚΘ§Β±AgClΩΣ ΦΉΣΜ·ΈΣAgI ±Θ§–κ¬ζΉψc(Ag+) c (I-)ΓίKsp(AgI)Θ§“ρ¥Υc (I-)Γί
mol/LΘ§ΒΈΦ”NaI»ή“ΚΘ§Β±AgClΩΣ ΦΉΣΜ·ΈΣAgI ±Θ§–κ¬ζΉψc(Ag+) c (I-)ΓίKsp(AgI)Θ§“ρ¥Υc (I-)Γί![]() =
=![]() =
=![]() ΓΝ10-11Θ§Ι ¥πΑΗΈΣΘΚΫΒΒΆΘΜ
ΓΝ10-11Θ§Ι ¥πΑΗΈΣΘΚΫΒΒΆΘΜ![]() ΓΝ10-11ΓΘ
ΓΝ10-11ΓΘ



| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ”…ΓΣC6H4ΓΣΓΔΓΣCH3ΓΔΓΣCH2ΓΣΓΔΓΣOHΥΡ÷÷‘≠Ή”Ά≈“ΜΤπΉι≥…ΒΡ τ”Ύ¥ΦάύΈο÷ ΒΡ÷÷άύ”–
A. 1÷÷ B. 2÷÷ C. 3÷÷ D. 4÷÷
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ≈πΤ’±ι¥φ‘Ύ”Ύ ΏΙϊ÷–Θ§ «Έ§≥÷Ι«ΒΡΫΓΩΒΚΆΗΤΓΔΝΉΓΔΟΨ’ΐ≥Θ¥ζ–ΜΥυ–η“ΣΒΡΈΔΝΩ‘ΣΥΊ÷°“ΜΓΘΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©≈π‘≠Ή”ΚΥΆβΒγΉ”’ΦΨίΉνΗΏΡή≤ψΒΡΖϊΚ≈ «__________Θ§’ΦΨίΉνΗΏΡήΦΕΒΡΒγΉ”‘Τ¬÷άΣΆΦ–ΈΉ¥ΈΣ___________ΓΘΦέ≤ψΒγΉ”ΒΡΙλΒά±μ¥ο Ϋ(ΒγΉ”≈≈≤ΦΆΦ)ΈΣ____________ΓΘ
Θ®2Θ©≈π‘≠Ή”ΒΡΒΎΕΰΒγΗΏΡή(I2)ΚΆΒΎ»ΐΒγάκΡή(I3)ΒΡ¥σ–ΓΙΊœΒΈΣΘΚI2______I3(ΧνΓΑ>Γ±ΜρΓΑ<Γ±),‘≠“ρ «____________ΓΘ
Θ®3Θ©BF3Ω…”Ο”Ύ÷Τ‘λΜπΦΐΒΡΗΏΡή»ΦΝœΓΘΤδΖ÷Ή”ΙΙ–ΆΈΣ___________Θ§“―÷ΣBF3Ζ÷Ή”÷–F‘≠Ή”ΚΆB‘≠Ή”≤…”ΟΆ§÷÷‘”Μ·ΖΫ ΫΈΣ____________Θ§BF3Ζ÷Ή”ΜΙ÷–¥φ‘Ύ¥σΠ–ΦϋΘ§Ω…”ΟΖϊΚ≈![]() ±μ Ψ(Τδ÷–m¥ζ±μ≤Έ”κ–Έ≥…¥σΠ–ΦϋΒΡ‘≠Ή” ΐΘ§n¥ζ±μ≤Έ”κ–Έ≥…¥σΠ–ΦϋΒΡΒγΉ” ΐΘ§»γ±ΫΖ÷Ή”÷–ΒΡ¥σΠ–ΦϋΩ…±μ ΨΈΣ
±μ Ψ(Τδ÷–m¥ζ±μ≤Έ”κ–Έ≥…¥σΠ–ΦϋΒΡ‘≠Ή” ΐΘ§n¥ζ±μ≤Έ”κ–Έ≥…¥σΠ–ΦϋΒΡΒγΉ” ΐΘ§»γ±ΫΖ÷Ή”÷–ΒΡ¥σΠ–ΦϋΩ…±μ ΨΈΣ![]() )Θ§‘ρΖ÷Ή”÷–ΒΡ¥σΠ–Φϋ”Π±μ ΨΈΣ____________ΓΘ
)Θ§‘ρΖ÷Ή”÷–ΒΡ¥σΠ–Φϋ”Π±μ ΨΈΣ____________ΓΘ
Θ®4Θ©≈πΥα(H3BO3)ΨßΧεΈΣ≤ψΉ¥ΫαΙΙΓΘΤδ÷–“Μ≤ψΒΡΫαΙΙΤ§ΕΈ»γΆΦ(a)Υυ ΨΘ§ΫΪ’β–©H3BO3Ζ÷Ή”ΨέΦ·‘Ύ“ΜΤπΒΡΉς”ΟΈΣ____________ΓΘ
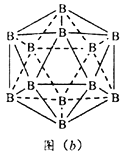
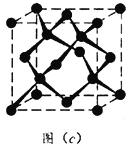
Θ®5Θ©ΨßΧε”Β”–Εύ÷÷±δΧεΘ§ΒΪΤδΜυ±ΨΫαΙΙΒΞ‘ΣΕΦ «”…≈π‘≠Ή”Ήι≥…ΒΡ’ΐΕΰ °ΟφΧεΘ§»γΆΦ(b)Θ§ΟΩΗωΕΞΒψΈΣΗω≈π‘≠Ή”Θ§ΙΙ≥…ΒΡ»ΐΫ«–ΈΨυΈΣΒ»±Ώ»ΐΫ«–ΈΘ§»τΗΟΫαΙΙΒΞ‘Σ÷–”–10Ηω‘≠Ή”ΈΣ10B(Τδ”ύΈΣ11B)Θ§Ρ«Ο¥ΗΟΫαΙΙΒΞ‘Σ”–_________÷÷≤ΜΆ§άύ–ΆΓΘ
Θ®6Θ©ΝΔΖΫΒΣΜ·≈π(BN)ΨßΧε”κΫπΗ’ ·ΨßΧεΜΞΈΣΒ»ΒγΉ”ΧεΘ§ΆΦ(c)ΈΣΫπΗ’ ·ΨßΧεΒΡΨßΑϊΘ§ΝΔΖΫΒΣΜ·≈πΨßΧεΩ…“‘»Γ≥ωΕύ÷÷ΨßΑϊΓΘΤδ÷–“Μ÷÷ΨßΑϊ÷–N»Ϊ≤ΩΈΜ”ΎΨßΑϊΧεΡΎΘ§‘ρB¥Π”Ύ____________ΈΜ÷ΟΓΘ

≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬Ν–Ζ¥”Π÷–Θ§ τ”Ύ»Γ¥ζΖ¥”ΠΒΡ «Θ® Θ©
A.““œ©‘ΎΩ’Τχ÷–»Φ…’
B.““Άι‘ΎΩ’Τχ÷–»Φ…’
C.““œ© Ι¬»Υ°Ά …Ϊ
D.““Άι”ꬻΤχ‘ΎΙβ’’œ¬―’…Ϊ±δ«≥
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΘ®1Θ©±ξΉΦΉ¥Ωωœ¬Θ§5.6 L AΤχΧεΒΡ÷ ΝΩ «15 g Θ§‘ρAΒΡœύΕ‘‘≠Ή”÷ ΝΩΈΣ___ΓΘ
Θ®2Θ©“ΜΕ®ΝΩΒΡ“ΚΧ§Μ·ΚœΈοXY2Θ§”κ“ΜΕ®ΝΩO2‘Ύ“ΜΟή±’»ίΤς÷–«ΓΚΟΆξ»ΪΖ¥”ΠΘΚXY2Θ®“ΚΘ©+3O2Θ®ΤχΘ©=XO2Θ®ΤχΘ©+2YO2Θ®ΤχΘ©Θ§ά以÷Ν±ξΉΦΉ¥ΩωΘ§≤βΒΟ»ίΤςΡΎΤχΧεΒΡΧεΜΐΈΣ6.72LΘ§ΟήΕ»ΈΣ2.5g/LΓΘ‘ρΘΚΜ·ΚœΈοXY2ΒΡΡΠΕϊ÷ ΝΩΈΣ________ΓΘ
Θ®3Θ©÷ ΝΩ÷°±»ΈΣ16:7:6ΒΡ»ΐ÷÷ΤχΧεSO2ΓΔCOΓΔNOΘ§Ζ÷Ή” ΐ÷°±»ΈΣ___________Θ§―θ‘≠Ή” ΐ÷°±»ΈΣ__________Θ§œύΆ§ΧθΦΰœ¬ΒΡΧεΜΐ÷°±»ΈΣ___________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΡ≥ΥαΒΡΥα Ϋ―ΈNaHY‘ΎΥ°»ή“Κ÷–Θ§HY-ΒΡΒγάκ≥ΧΕ»–Γ”ΎHY-ΒΡΥ°Ϋβ≥ΧΕ»ΓΘ”–ΙΊΒΡ–π ω÷–Θ§’ΐ»ΖΒΡ «
A. H2YΒγάκΖΫ≥Χ ΫΈΣΘΚH2Y+H2O![]() HYΘ≠+H3O+
HYΘ≠+H3O+
B. ≥ΘΈ¬œ¬Θ§ΗΟΥα Ϋ―ΈΒΡΥ°»ή“Κ÷–»ή“Κ÷–Θ§ΗςάκΉ”≈®Ε»¥σ–ΓΙΊœΒΈΣ:cΘ®Na+Θ©>cΘ®Y2-Θ©>cΘ®HY-Θ©>cΘ®OH-Θ©>cΘ®H+Θ©
C. ≥ΘΈ¬œ¬,Υα Ϋ―ΈNaHY ΒΡΥ°»ή“Κ≥ Υα–‘
D. HYΘ≠ΒΡΥ°ΫβΖΫ≥Χ ΫΈΣΘΚHYΘ≠+ H2O![]() H3O++Y2Θ≠
H3O++Y2Θ≠
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ÷Μ”Ο“Μ÷÷ ‘ΦΝΨΆΩ…“‘Φχ±π““Υα»ή“ΚΓΔΤœΧ―Χ«»ή“ΚΓΔΒμΖέ»ή“ΚΘ§ΗΟ ‘ΦΝ «Θ® Θ©
A.NaOH»ή“Κ
B.Na2CO3»ή“Κ
C.ΒβΥ°
D.–¬÷Τ«β―θΜ·Ά≠
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΈΣ≥ΐ»Ξ¥÷―Έ÷–ΒΡCa2ΘΪΓΔMg2ΘΪΓΔSO![]() “‘ΦΑΡύ…≥Β»‘”÷ Θ§Ρ≥Ά§―ß…ηΦΤΝΥ“Μ÷÷÷Τ±ΗΨΪ―ΈΒΡ Β―ιΖΫΑΗΘ§≤Ϋ÷η»γœ¬ΘΚ(”Ο”Ύ≥ΝΒμΒΡ ‘ΦΝ…‘ΙΐΝΩ)
“‘ΦΑΡύ…≥Β»‘”÷ Θ§Ρ≥Ά§―ß…ηΦΤΝΥ“Μ÷÷÷Τ±ΗΨΪ―ΈΒΡ Β―ιΖΫΑΗΘ§≤Ϋ÷η»γœ¬ΘΚ(”Ο”Ύ≥ΝΒμΒΡ ‘ΦΝ…‘ΙΐΝΩ)
≥Τ»Γ¥÷―Έ![]()
![]()
![]()
![]()
![]() ¬Υ“Κ
¬Υ“Κ![]()
![]() ΨΪ―Έ
ΨΪ―Έ
Θ®1Θ©ΒΎΔΌ≤Ϋ÷–Θ§≤ΌΉςA «________Θ§ΒΎΔί≤Ϋ÷–Θ§≤ΌΉςB «________ΓΘ
Θ®2Θ©ΒΎΔή≤Ϋ÷–Θ§–¥≥ωœύ”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ(ΦΌ…η¥÷―Έ»ή“Κ÷–Ca2ΘΪΒΡ÷ς“Σ¥φ‘Ύ–Έ ΫΈΣCaCl2)_______ΓΘ
Θ®3Θ©»τœ»”Ο―ΈΥαΒςpH‘ΌΙΐ¬ΥΘ§ΫΪΕ‘ Β―ιΫαΙϊ≤ζ…ζ”ΑœλΘ§Τδ‘≠“ρ «__________________ΓΘ
Θ®4Θ©≈–ΕœBaCl2“―ΙΐΝΩΒΡΖΫΖ® «___________________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΒΰΒΣΜ·ΡΤΘ®NaN3Θ© «“Μ÷÷”Π”ΟΙψΖΚΒΡΜ·ΙΛ≤ζΤΖΘ§Ω…”Ο”ΎΚœ≥…ΩΙ…ζΥΊΆΖφΏΨζΥΊ“©ΈοΒΡ÷–ΦδΧεΘ§Τϊ≥ΒΑ≤»ΪΤχΡ“Β»ΓΘΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Δώ. Β―ι “÷Τ±ΗNaN3
Υ°Κœκ¬(N2H4ΓΛH2O) ”κ―«œθΥαΦΉθΞ(CH3ONO)‘Ύ«β―θΜ·ΡΤ¥φ‘Ύœ¬÷Τ±ΗNaN3Θ§ΤδΖ¥”ΠΉΑ÷Ο»γΆΦΥυ ΨΘΚ
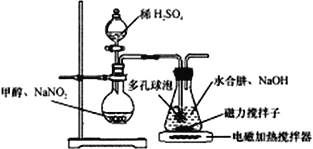
“―÷ΣΘΚ2CH3OH+2NaNO2+H2SO4Γζ2CH3ONO+ Na2SO4+2H2OΘΜ NaN3Έό…ΪΈόΈΕΘ§ΈΔ»ή”Ύ¥ΦΓΔ»ή”ΎΥ°
Θ®1Θ©N2H4ΒΡΒγΉ” ΫΈΣ_______________ΘΜNaN3ΨßΧε÷–“θάκΉ””κ―τάκΉ”Ηω ΐ±»ΈΣ______________ΓΘ
Θ®2Θ©ΉΑ÷Ο÷–ΕύΩΉ«ρ≈ίΒΡΉς”Ο «___________________ΓΘ
Θ®3Θ©ΉΕ–ΈΤΩ÷–Υ°Κœκ¬”κ―«œθΥαΦΉθΞ‘Ύ30Γφ ±Ω…“‘Ζ¥”Π…ζ≥…ΒΰΒΣΥαΡΤΓΔΦΉ¥ΦΒ»Έο÷ Θ§–¥≥ωΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ_______________________________ΓΘ
Δρ.ΜΊ ’ΦΉ¥Φ
ΫΪ÷Τ±ΗΖ¥”ΠΚσΥυΒΟΜλΚœ»ή“ΚΦ”»κ…’ΤΩ÷–Θ§Α¥’’œ¬ΆΦΥυ ΨΉΑ÷ΟΫχ––Φθ―Ι’τΝσΓΘ
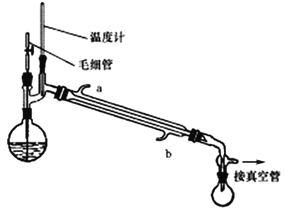
“―÷ΣΘΚ
Έο÷ | CH3OH | N2H4 | NaN3 |
Ζ–Βψ/Γφ | 64.7 | 113.5 | 300 |
NaN3‘Ύ40Γφ ±Ζ÷Ϋβ
Θ®4Θ© Β―ι ±άδΡΐΙή÷–ά以հ“ΣΓΑbΫχa≥ωΓ±‘≠“ρ «______________________ΓΘ
Θ®5Θ©ΦΉ¥ΦΜΊ ’ ±–η”ΟΦθ―Ι’τΝσΒΡ‘≠“ρ «________________________ΓΘ
Θ®6Θ©œ¬Ν–”–ΙΊΟΪœΗΙήΒΡΉς”ΟΥΒΖ®’ΐ»ΖΒΡ «_______________ΓΘ
A.ΤΫΚβ‘≤ΒΉ…’ΤΩΡΎΆβ―Ι B.ΉςΈΣΤχΜ·÷––ΡΘ§ Ι’τΝσΤΫΈ»
C.±ήΟβ“ΚΧεΙΐ»»Εχ±©Ζ– D.άδΡΐΜΊΝςΉς”Ο
Δσ.≤ζΤΖΧα»ΓΦΑ¥ΩΕ»≤βΕ®
ΫΪ’τΝσΚσΥυΒΟΡΗ“ΚΫΒΈ¬ΫαΨßΘ§Ιΐ¬ΥΒΟNaN3 ΣΤΖΘΜ‘Ό”Ο»ΞάκΉ”Υ°÷ΊΫαΨßΒΟNaN3≤ζΤΖ≤Δ”ΟΒβΝΩΖ®≤βΕ®≤ζΤΖ¥ΩΕ»ΓΘ»Γ≤ζΤΖ6.50gΦ”»κΉψΝΩ»ΞάκΉ”Υ°÷–»ήΫβΘ§≤ΔΦ”»κ ΝΩœΓΝρΥαΥαΜ·ΘΜœρΜλΚœ“Κ÷–Φ”»κ20.00mL 1.00molΓΛL-lKMnO4»ή“ΚΘ§»ή“Κ≥ ΉœΚλ…ΪΘΜ‘ΌΦ”»κΉψΝΩKI»ή“ΚœϊΚΡΙΐΝΩΒΡKMnO4»ή“ΚΘΜΤδΚσ”Ο0.100molΓΛL-lNa2S2O3±ξΉΦ»ή“ΚΒΈΕ®Υυ≤ζ…ζΒΡI2Θ§œϊΚΡNa2S2O3»ή“Κ30.00mLΓΘ
Θ®7Θ© Β―ιΥυΒΟ≤ζΤΖΒΡ¥ΩΕ»ΈΣ______________________ΓΘ
“―÷ΣΘΚΔΌ≤ζΤΖ÷–‘”÷ ≤Μ≤Έ”κΖ¥”ΠΘΜ
ΔΎ≤βΕ®Ιΐ≥Χ÷–ΖΔ…ζΒΡΖ¥”ΠΘΚ
10NaN3+2KMnO4+8H2SO4==2MnSO4+K2SO4+5Na2SO4+8H2O+15N2ΓϋΘΜ
10KI+2KMnO4+8H2SO4==2MnSO4+6K2SO4+8H2O+5I2ΘΜ
I2+2Na2S2O3==2NaI +Na2S4O6ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com