
��������ѧ��ѧʵ���ҵij���ҩƷ���������Т����ԡ�����ˮ�ԡ�����ˮ�ԡ���ǿ�����ԡ��ݴ����ã��뽫���������Ӧ�ĺ����ϣ�
(1)п��ϡH2SO4��H2________��
(2)Ũ�����������________��
(3)Ũ���������ǵ�̿��ʵ��(�����ʵ��)________��
(4)ʵ�������Ҵ��ͱ�������ȡ��������________��
(5)����������ˮ��________��
(6)��ά�ص�ˮ��________��
(7)Ũ������ͭ�ķ�Ӧ________��
(8)Ũ����ʹʪ��ʯ����ֽ��죬�����ֱ��________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������Ż𣬲�����H2O���ɱ�����ԭ����_____________________________________��
ͨ����__________����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ǻ��õļ����Ԫ�أ��Ƽ��仯�������������������й㷺��Ӧ�á�
������м��㣺
(1)��������(NaN3)��ײ����ȫ�ֽ�����ƺ͵������ʿ�Ӧ����������ȫ���ҡ�������40.32 L(��״����)������������Ҫ��������________g��
(2)���غϽ���ں˷�Ӧ���������Ƚ���Һ��5.05 g���غϽ�����200 mLˮ����0.075 mol������
�ټ�����Һ�����������ӵ����ʵ���Ũ��(������Һ����仯)��
�ڼ��㲢ȷ�������غϽ�Ļ�ѧʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����SO2����������ȷ����(����)
A��SO2ͨ����ˮ����Һ��ɫ�����Լ���
B�����������������ˮ���ȶ���������
C��SO2����ͨ��NaOH��Һһ���õ�Na2SO3
D��S��SO2��SiO2�������ʾ�����NaOH��Һ��Ӧ������������ijЩ�ᷴӦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)���������У����γ��������______��
A���������� B�����ȴ���
C��������̼ D������
(2)�������¼��ִ�ʩ���ٶ�ȼ��úʱ������β�����г�������������ԭú��ȼ�ϣ���ȼúʱ���������������ܿ��������Դ�������ܼ�����������Ĵ�ʩ��______��
A���٢ڢ� B���ڢۢ�
C���٢ڢ� D���٢ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ȤС����ʵ������ͭ������Ϊԭ�ϣ����ö��ַ�����ȡ����ͭ���Ʊ��������£�
����һ
(1)Ũ�����Լ�ƿ���ʺ����ϵı�ǩ��________(�����)��

(2)��ͬѧȡ6.4 gͭƬ��10 mL 18 mol·L��1Ũ���ᣬ�����Թ��й���ʱ���֣�ͭ���ȵ�Ũ���ᷴӦ��û�еõ�Ԥ�ڵ���ɫ��Һ���������Թܵײ������Ұ�ɫ��������ͬѧΪ����֤���лҰ�ɫ��������Ҫ�ɷ֣��������ʵ�飺
ʵ�鲽�裺�㵹���ϲ�Һ��������ûҰ�ɫ�Ĺ����м�����������ˮ���ӱ߽��衣
ʵ������_________________________________��
ʵ����ۣ����ûҰ�ɫ����Ļ�ѧʽΪ__________��
(3)�һ��۲쵽���ȹ����У��Թ��ڱ��ϲ�������������ɫ�������ʣ��������ȣ�����ɫ��������������������Ũ�������ʧ������ɫ������ʧ��ԭ����(�û�ѧ����ʽ�ش�)________________________________________________________________________��
ֱ�����Ӧ��ϣ������Թ��л���ͭƬʣ�࣬�Ҹ����Լ���ѧ�Ļ�ѧ֪ʶ����Ϊ�Թ��л�������ʣ�ࡣ��������Ϊ��������________________________________________________________________________��
������
(4)��ͬѧ��Ϊ����Ƶ�ʵ�鷽�����ã����Լ���Ƶ�˼·��2Cu��O2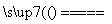 2CuO��CuO��H2SO4===CuSO4��H2O��
2CuO��CuO��H2SO4===CuSO4��H2O��
�Աȼķ���������Ϊ��ͬѧ���ŵ��Ǣ�________________________________________________________________________��
��________________________________________________________________________��
������
(5)��ͬѧȡһͭƬ��ϡ��������Թ��У��������е���˫��ˮ������Һ����ɫ��д����Ӧ�Ļ�ѧ����ʽ________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������һ����Ҫ�Ļ�����Ʒ��ij��ȤС�����Ʊ���������ƾ���(Na2S2O3·5H2O)��
��.[��������]
(1)Na2S2O3·5H2O����ɫ�����壬������ˮ����ϡ��Һ��BaCl2��Һ����������ɡ�
(2)��Na2CO3��Na2S�����Һ��ͨ��SO2���Ƶ�Na2S2O3�����ò�Ʒ����������Na2SO3��Na2SO4��
(3)Na2SO3�ױ�������BaSO3������ˮ��������ϡHCl��
��.[�Ʊ���Ʒ]
ʵ��װ����ͼ��ʾ(ʡ�Լг�װ��)��
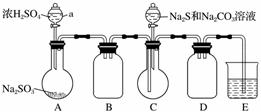
ʵ�鲽�裺
(1)���װ�������ԣ���ͼʾ�����Լ���
����a��������________��E�е��Լ���________(ѡ��������ĸ���)��
A��ϡH2SO4
B��NaOH��Һ
C������NaHSO3��Һ
(2)����C����ƿ����Na2S��Na2CO3�����Һ������A����ƿ�μ�ŨH2SO4��
(3)��Na2S��Na2CO3��ȫ���ĺ�����Ӧ������C�л�����Һ��__________(��д��������)���ᾧ�����ˡ�ϴ�ӡ�����õ���Ʒ��
��.[̽���뷴˼]
(1)Ϊ��֤��Ʒ�к���Na2SO3��Na2SO4����С�����������ʵ�鷽�����뽫��������������
(�����Լ���ϡHNO3��ϡH2SO4��ϡHCl������ˮ��ѡ��)
ȡ������Ʒ���ϡ��Һ���μ�����BaCl2��Һ���а�ɫ�������ɣ�________________��������δ��ȫ�ܽ⣬���д̼�����ζ��������������ȷ����Ʒ�к���Na2SO3��Na2SO4��
(2)Ϊ����װ��C������Na2SO4�������ڲ��ı�ԭ��װ�õĻ����϶�ʵ�鲽��(2)�����˸Ľ����Ľ���IJ�����
________________________________________________________________________��
(3)Na2S2O3·5H2O���ܽ�����¶����������������ò�Ʒͨ��________________�����ᴿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���л�����ķ����У�����ԭ�Ӷ�����ͬһƽ����ǣ� ��
A�������� B����ȩ C���������ױ� D��������ϩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и����ʵľ����У�����������ͬ����(����)
A��O2��SiO2 B��NaI��I2
C��CO2��H2O D��CCl4��NaCl
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com