
ŅŃÖŖijÖÖČ¼ĮĻŗ¬ÓŠĢ¼”¢Ēā”¢Ńõ3ÖÖŌŖĖŲ”£ĪŖĮĖ²ā¶ØÕāÖÖČ¼ĮĻÖŠĢ¼ŗĶĒāĮ½ÖÖŌŖĖŲµÄÖŹĮæ±Č£¬æɽ«ĘųĢ¬Č¼ĮĻ·ÅČė×ćĮæµÄŃõĘųÖŠČ¼ÉÕ£¬²¢Ź¹²śÉśµÄĘųĢåČ«²æĶØČėČēĶ¼ĖłŹ¾µÄ×°ÖĆ£¬µĆµ½ČēĻĀ±ķĖłĮŠµÄŹµŃé½į¹ū(¼ŁÉč²śÉśµÄĘųĢåĶźČ«±»ĪüŹÕ)

|
|
ŹµŃéĒ° |
ŹµŃéŗó |
|
(øÉŌļ¼Į£«UŠĪ¹Ü)µÄÖŹĮæ |
101.1 g |
102.9 g |
|
(ŹÆ»ŅĖ®£«¹ćæŚĘæ)µÄÖŹĮæ |
312.0 g |
314.2 g |
øł¾ŻŹµŃ鏿¾ŻĒó£ŗ
(1)ŹµŃéĶź±Ļŗó£¬Éś³ÉĪļÖŠĖ®ÖŹĮæĪŖ________g£¬¼ŁÉč¹ćæŚĘæĄļÉś³ÉŅ»ÖÖÕżŃĪ£¬ĘäÖŹĮæĪŖ________g£»
(2)Éś³ÉµÄĖ®ÖŠĒāŌŖĖŲµÄÖŹĮæĪŖ________g£»
(3)Éś³ÉµÄ¶žŃõ»ÆĢ¼ÖŠĢ¼ŌŖĖŲµÄÖŹĮæĪŖ________g£»
(4)øĆČ¼ĮĻÖŠĢ¼ŌŖĖŲÓėĒāŌŖĖŲµÄÖŹĮæ±ČĪŖ________£»
(5)ŅŃÖŖÕāÖÖČ¼ĮĻµÄĆæøö·Ö×ÓÖŠŗ¬ÓŠŅ»øöŃõŌ×Ó£¬ŌņøĆČ¼ĮĻµÄ·Ö×ÓŹ½ĪŖ________£¬½į¹¹¼ņŹ½ĪŖ________”£
(1)1.8””5””(2)0.2 ””(3)0.6””(4)3”Ć1 (5)CH4O””CH3OH
”¾½āĪö”æ(1)m(H2O)£½102.9 g£101.1 g£½1.8 g
m(CO2)£½314.2 g£312.0 g£½2.2 g
Ōņn(CaCO3)£½n(CO2)£½0.05 mol
m(CaCO3)£½5 g
(2)m(H)£½m(H2O)”Į2/18£½1.8 g”Į2/18£½0.2 g
(3)m(C)£½m(CO2)”Į12/44£½2.2 g”Į12/44£½0.6 g
(4)m(C)”Ćm(H)£½0.6 g”Ć0.2 g£½3”Ć1
(5)øĆČ¼ĮĻ·Ö×ÓÖŠC”¢HµÄŌ×ÓøöŹż±ČĪŖ£ŗn(C)”Ćn(H)£½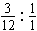 £½1”Ć4
£½1”Ć4
¾ŻĢ¼ĖļŪµÄŌŌņÖŖ£¬µ±ÓŠ»śĪļ·Ö×ÓÖŠµÄĢ¼ĒāŌ×ÓøöŹż±ČĪŖ1”Ć4Ź±£¬·Ö×ÓÖŠÖ»ÄÜŗ¬CH4£¬¶ų²»ÄÜĪŖCH4µÄÕūŹż±¶£¬ÓÖŅņĪŖĆæøö·Ö×ÓÖŠŗ¬ÓŠŅ»øöŃõŌ×Ó£¬ŌņøĆČ¼ĮĻµÄ·Ö×ÓŹ½ĪŖCH4O£¬½į¹¹¼ņŹ½ĪŖCH3OH”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
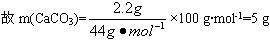
| ŹµŃéĒ° | ŹµŃéŗó |
(øÉŌļ¼Į+UŠĪ¹Ü)µÄÖŹĮæ | ||
(ŹÆ»ŅĖ®+¹ćæŚĘæ)µÄÖŹĮæ |
øł¾ŻŹµŃ鏿¾ŻĶź³ÉĻĀĮŠĪŹĢā£ŗ
(1)ŹµŃéĶź±Ļŗó£¬Éś³ÉĪļÖŠĖ®µÄÖŹĮæĪŖ_______________g£¬¼ŁÉč¹ćæŚĘæĄļÉś³ÉŅ»ÖÖÕżŃĪ£¬ĘäÖŹĮæĪŖ_______________ g”£
(2)Éś³ÉµÄĖ®ÖŠĒāŌŖĖŲµÄÖŹĮæĪŖ_______________ g”£
(3)Éś³ÉµÄ¶žŃõ»ÆĢ¼ÖŠĢ¼ŌŖĖŲµÄÖŹĮæĪŖ_______________ g”£
(4)øĆČ¼ĮĻÖŠĢ¼ŌŖĖŲÓėĒāŌŖĖŲµÄÖŹĮæ±ČĪŖ_______________”£
(5)ŅŃÖŖÕāÖÖČ¼ĮĻµÄĆæøö·Ö×ÓÖŠÖ»ÄÜŗ¬ÓŠŅ»øöŃõŌ×Ó£¬ŌņøĆČ¼ĮĻµÄ·Ö×ÓŹ½ĪŖ______________,½į¹¹¼ņŹ½ĪŖ_______________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖijÖÖČ¼ĮĻŗ¬ÓŠĢ¼”¢Ēā”¢ŃõČżÖÖŌŖĖŲ”£ĪŖĮĖ²ā¶ØÕāÖÖČ¼ĮĻÖŠĢ¼ŗĶĒāĮ½ÖÖŌŖĖŲµÄÖŹĮæ±Č£¬æɽ«ĘųĢ¬Č¼ĮĻ·ÅČė×ćĮæµÄŃõĘųÖŠČ¼ÉÕ£¬²¢Ź¹²śÉśµÄĘųĢåČ«²æĶØČėČēÉĻĶ¼ĖłŹ¾µÄ×°ÖĆ£¬µĆµ½ČēĻĀ±ķĖłĮŠµÄŹµŃé½į¹ū£Ø¼ŁÉč²śÉśµÄĘųĢåĶźČ«±»ĪüŹÕ£©£ŗ
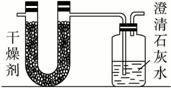
| ŹµŃéĒ° | ŹµŃéŗó | |
| £ØøÉŌļ¼Į+UŠĪ¹Ü£©µÄÖŹĮæ | 101.1 g | 102.9 g |
| £ØŹÆ»ŅĖ®+¹ćæŚĘ棩µÄÖŹĮæ | 312.0 g | 314.2 g |
øł¾ŻŹµŃ鏿¾ŻĶź³ÉĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŹµŃéĶź±Ļŗó£¬Éś³ÉĪļÖŠĖ®µÄÖŹĮæĪŖ_______________g£¬¼ŁÉč¹ćæŚĘæĄļÉś³ÉŅ»ÖÖÕżŃĪ£¬ĘäÖŹĮæĪŖ_______________ g”£
£Ø2£©Éś³ÉµÄĖ®ÖŠĒāŌŖĖŲµÄÖŹĮæĪŖ_______________ g”£
£Ø3£©Éś³ÉµÄ¶žŃõ»ÆĢ¼ÖŠĢ¼ŌŖĖŲµÄÖŹĮæĪŖ_______________ g”£
£Ø4£©øĆČ¼ĮĻÖŠĢ¼ŌŖĖŲÓėĒāŌŖĖŲµÄÖŹĮæ±ČĪŖ_______________”£
£Ø5£©ŅŃÖŖÕāÖÖČ¼ĮĻµÄĆæøö·Ö×ÓÖŠÖ»ÄÜŗ¬ÓŠŅ»øöŃõŌ×Ó£¬ŌņøĆČ¼ĮĻµÄ·Ö×ÓŹ½ĪŖ______________,½į¹¹¼ņŹ½ĪŖ_______________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗĶ¬²½Ģā ĢāŠĶ£ŗŹµŃéĢā
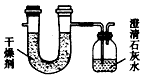

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ŹµŃéĒ° | ŹµŃéŗó |
£ØøÉŌļ¼Į+UŠĪ¹Ü£©µÄÖŹĮæ | 101.1 g | 102.9 g |
£ØŹÆ»ŅĖ®+¹ćæŚĘ棩µÄÖŹĮæ | 312.0 g | 314.2 g |
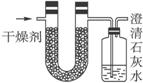
øł¾ŻŹµŃ鏿¾ŻĒó£ŗ
£Ø1£©ŹµŃéĶź±Ļŗó£¬Éś³ÉĪļÖŠĖ®µÄÖŹĮæĪŖ_________g”£¼ŁÉč¹ćæŚĘæĄļÉś³ÉŅ»ÖÖÕżŃĪ£¬ĘäÖŹĮæĪŖ_________g”£
£Ø2£©øĆČ¼ĮĻÖŠĢ¼ŌŖĖŲÓėĒāŌŖĖŲµÄÖŹĮæ±ČĪŖ_________”£
£Ø3£©ŅŃÖŖÕāÖÖČ¼ĮĻµÄĆæøö·Ö×ÓÖŠŗ¬ÓŠŅ»øöŃõŌ×Ó£¬ŌņøĆČ¼ĮĻµÄ·Ö×ÓŹ½ĪŖ_________£¬½į¹¹¼ņŹ½ĪŖ_________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com