
װ �� |  |  |  |
| �� �� | ����A�����ܽ� | C���������� | A����������� |
| ������Ӧʽ |




| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
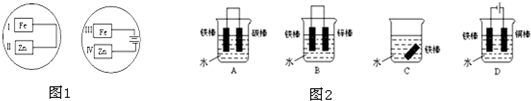
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
װ �� |
 |
 |
 |
| �� �� | ����A�����ܽ� | C���������� | A����������� |
| ������Ӧʽ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡտ���б�ҵ����в������ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
NiSO4•6H2O��һ����ɫ������ˮ�ľ��壬�㷺���ڻ�ѧ������������صȣ����ɵ�Ʒ������������⣬�����У�Cu��Zn��Fe��Cr��Ԫ�صĻ��������ʣ�Ϊԭ�ϻ�á��йع����������£�

��1���������м�H2SO4������Ҫ��ֽ��裬��Ŀ���� ��
��2����Na2S��Ŀ���dz�ȥͭ��п�����ʣ���д����ȥCu2+�����ӷ���ʽ ��
��3����6%��H2O2ʱ���¶Ȳ��ܹ��ߣ���Ŀ���� ��
��4��������������H2O2�������������NaOH����pHֵ2��4��Χ�������������������������������У�����������NaClO3���棬��NaClO3����Fe2+�����ӷ���ʽΪ ��
��5��������������ҺIII�����ʵ���Ҫ�ɷ��ǣ� ��
��6������I�������¹��̣����ˣ���H2SO4�ܽ⣬ �� �����ˡ�ϴ�ӻ�ò�Ʒ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ����12�½��Լ�⻯ѧ�Ծ��������棩 ���ͣ������
����12�֣�
��1����CO2Ϊ̼Դ��ȡ��̼�л���һֱ�ǻ�ѧ������о��ȵ㣬CO2������ȡ��̼���ķ�Ӧ���£�
��ӦI��CO2(g)+3H2(g)=CH3OH(g)+H2O(g) ��H=��49.0kJ/mol
��ӦII��2CO2(g)+6H2(g)=CH3CH2OH(g)+3H2O(g) ��H=��173.6kJ/mol
д����CH3OH(g)�ϳ�CH3CH2OH(g)�ķ�Ӧ���Ȼ�ѧ����ʽ_______________
��2��������أ�K2FeO4��������һ����Ҫ��������м�ǿ��������
�ٵ�ⷨ�ǹ�ҵ���Ʊ�K2FeO4��һ�ַ���������Ϊ�����������������Һ��Ȼ����������Һ�м���KOH�����ʱ����������Ӧ����FeO42-���õ缫��ӦʽΪ_________________
����MnO2��Zn������ƣ�K2FeO4��ZnҲ������ɼ��Ե�أ�K2FeO4�ڵ�������������ϣ���缫��ӦʽΪFeO42-+3e-+4H2O=Fe(OH)3+5OH-����õ���ܷ�Ӧ�����ӷ���ʽΪ__________________
��3��amol FeS��bmol FeOͶ�뵽VL��C mol/L��������Һ�г�ַ�Ӧ����NO���壬���ó�����Һ�ɷֿɿ�����Fe(NO3)3��H2SO4�Ļ��Һ����Ӧ��δ����ԭ���������Ϊ_____
�٣�a+b����63g �ڣ�a+b����189g �ۣ�a+b��mol ��VC�� mol
mol
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com