
| A£® | ĀĮÄĘŗĻ½šĶ¶Čėµ½×ćĮæĀČ»ÆĶČÜŅŗÖŠ£¬æĻ¶ØÓŠĒāŃõ»ÆĶ³ĮµķŅ²æÉÄÜÓŠĶĪö³ö | |
| B£® | ĀĮÄĘŗĻ½šČōĶ¶ČėŅ»¶ØµÄĖ®ÖŠæɵĆĪŽÉ«ČÜŅŗ£¬Ōņn£ØAl£©”Ün£ØNa£© | |
| C£® | ĀĮÄĘŗĻ½šĶ¶Čė×ćĮæĖ®ÖŠ£¬Čō·Å³öµÄH2Ō½¶ą£¬ŌņĀĮµÄÖŹĮæ·ÖŹżŌ½“ó | |
| D£® | ĀĮÄĘŗĻ½šĶ¶Čė×ćĮæŃĪĖįÖŠ£¬Čō·Å³öµÄH2Ō½¶ą£¬ŌņĀĮµÄÖŹĮæ·ÖŹżŌ½“ó |
·ÖĪö A£®ÄĘŗĶĖ®·“Ӧɜ³ÉĒāŃõ»ÆÄĘŗĶĒāĘų£¬ĀĮŗĶĒāŃõ»ÆÄĘ·“Ó¦£¬ĀČ»ÆĶŗĶĒāŃõ»ÆÄĘ·“Ӧɜ³ÉĒāŃõ»ÆĶ³Įµķ£¬ĀĮ¹żĮæÄÜÖĆ»»³öĶ£»
B£®øłĀĮÄĘŗĻ½šČōĶ¶ČėŅ»¶ØµÄĖ®ÖŠæɵĆĪŽÉ«ČÜŅŗ£¬ĖµĆ÷ÄĘŗĶĖ®·“Ӧɜ³ÉµÄĒāŃõ»ÆÄĘ×ćŅŌ½«½šŹōĀĮČܽā£»
C£®ÄĘČÜÓŚĖ®Éś³ÉĒāŃõ»ÆÄĘŗĶĒāĘų£¬ĀĮŗĶĖ®²»·“Ó¦£¬ĒāŃõ»ÆÄĘÓėĀĮ·“Ӧɜ³ÉĘ«ĀĮĖįÄĘŗĶĒāĘų£¬ĀĮÄĘŗĻ½šĶ¶Čė×ćĮæĖ®ÖŠ£¬Čō·Å³öµÄH2Ō½¶ą£¬ŌņĀĮµÄÖŹĮæ·ÖŹżŌ½Š”£»
D£®µČÖŹĮæµÄ½šŹōÄĘŗĶ½šŹōĀĮ£¬½šŹōAl²śÉśµÄĒāĘųĮæ¶ą£¬ĀĮÄĘŗĻ½šÓėŃĪĖį·“Ó¦·Å³öµÄH2Ō½¶ą£¬ĀĮµÄÖŹĮæ·ÖŹżŌ½“ó£»
½ā“š ½ā£ŗA£®ĀĮÄĘŗĻ½šĶ¶Čėµ½×ćĮæĀČ»ÆĶČÜŅŗÖŠ£¬ÄĘŗĶĖ®·“Ó¦2Na+2H2O=2NaOH+H2”ü£¬Éś³ÉµÄĒāŃõ»ÆÄĘæÉŅŌŗĶĀČ»ÆĶ·“Ӧɜ³ÉĒāŃõ»ÆĶ³Įµķ£¬2Al+2NaOH+2H2O=2NaAlO2+3H2”ü£¬Čōn£ØAl£©£¾n£ØNa£©£¬½šŹōĀĮŅ²æÉÄÜ»įÖĆ»»³öĀČ»ÆĶÖŠµÄ½šŹōĶ£¬¹ŹAÕżČ·£»
B£®ĀĮÄĘŗĻ½šČōĶ¶ČėŅ»¶ØµÄĖ®ÖŠ·¢Éś2Na+2H2O=2NaOH+H2”ü£¬2Al+2NaOH+2H2O=2NaAlO2+3H2”ü£¬µ±n£ØAl£©”Ün£ØNa£©Ź±£¬µĆµ½ĪŽÉ«ČÜŅŗ£¬¹ŹBÕżČ·£»
C£®ĀĮÄĘŗĻ½šČōĶ¶ČėŅ»¶ØµÄĖ®ÖŠ·¢Éś2Na+2H2O=2NaOH+H2”ü£¬2Al+2NaOH+2H2O=2NaAlO2+3H2”ü£¬ĀĮŗĶĖ®²»·“Ó¦£¬Čō·Å³öµÄH2Ō½¶ą£¬ĪŖn£ØAl£©£¼n£ØNa£©Ź±£¬ŌņĀĮµÄÖŹĮæ·ÖŹżŌ½Š”£¬¹ŹC“ķĪó£»
D£®øł¾Ż½šŹōÄĘŗĶ½šŹōĀĮŗĶĖį·“Ӧɜ³ÉĒāĘųĮæµÄ¹ŲĻµ£ŗ2Al”«3H2”ü£¬2Na”«H2”ü£¬µČÖŹĮæµÄ½šŹōÄĘŗĶ½šŹōĀĮ£¬Ōņ½šŹōAl²śÉśµÄĒāĘųĮæ¶ą£¬ĖłŅŌ·Å³öµÄH2Ō½¶ą£¬ŌņĀĮµÄÖŹĮæ·ÖŹżŌ½“󣬹ŹDÕżČ·£»
¹ŹŃ”C£®
µćĘĄ ±¾Ģā×ŪŗĻæ¼²éÄĘ”¢ĀĮµÄŠŌÖŹ£¬×¢Ņāøł¾Ż2Na+2H2O=2NaOH+H2”ü£¬2Al+2NaOH+2H2O=2NaAlO2+3H2”ü·½³ĢŹ½½ųŠŠÅŠ¶ĻŹĒ½ā“š¹Ų¼ü£¬ĢāÄæÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ČōøıäÓ°Ļģ»ÆŃ§Ę½ŗāµÄĢõ¼žÖ®Ņ»£¬Ę½ŗāĻņÄܹ»Ź¹ÕāÖÖøıä¼õČõµÄ·½ĻņŅĘ¶Æ | |
| B£® | “ļµ½»ÆŃ§Ę½ŗāŹ±£¬ø÷×é·ÖµÄÅØ¶Č²»ŌŁøı䣬·“Ó¦Ķ£Ö¹ | |
| C£® | »ÆŃ§Ę½ŗāŅĘ¶Æ£¬»ÆŃ§Ę½ŗā³£Źż²»Ņ»¶Øøıä |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | pHĻąĶ¬µÄ¢ŁCH3COONa””¢ŚNaHCO3””¢ŪNaClOČżÖÖČÜŅŗµÄc£ØNa+£©£ŗ¢Ū£¾¢Ś£¾¢Ł | |
| B£® | 0.2mol•L-1 CH3COOHČÜŅŗÓė0.1mol•L-1NaOHČÜŅŗµČĢå»ż»ģŗĻ2c£ØH+£©-2c£ØOH-£©=c£ØCH3COO-£©-c£ØCH3COOH£© | |
| C£® | ŅŃÖŖµēĄėĘ½ŗā³£Źż£ŗH2CO3£¾HClO£¾HCO3-£¬ĻņNaClOČÜŅŗÖŠĶØČėÉŁĮæCO2£ŗ2ClO-+CO2+H2OØT2HClO+CO32- | |
| D£® | ³£ĪĀĻĀ£¬Ļņ0.1mol/L NH4HSO4ČÜŅŗÖŠµĪ¼ÓNaOHČÜŅŗÖĮÖŠŠŌc£ØNa+£©£¾c£ØNH4+£©£¾c£ØSO42-£©£¾c£ØOH-£©=c£ØH+£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŅŃÖŖC2H2£Øg£©+$\frac{5}{2}$O2£Øg£©”ś2CO2£Øg£©+H2O£Øl£©”÷H=-1300KJ/mol µ±ÓŠ5NAøöµē×Ó×ŖŅĘŹ±Äܷųö650KJµÄČČĮæ | |
| B£® | ÓŠ28gŅŅĻ©Óė±ūĻ©»ģŗĻĘųĢ壬Ęäŗ¬Ō×Ó×ÜŹżĪŖ3NAøö | |
| C£® | ÓŠ·“Ó¦H2£Øg£©+I2£Øg£©?2HI£Øg£©”÷H=-QHJ/molŌŚ298K 101KPaĻĀĻņŅ»ĆܱÕČŻĘ÷ÖŠ³äČėNAøöH2ŗĶNAøöI2£¬³ä·Ö·“Ó¦¹²·Å³öČČĮæĪŖQKJ | |
| D£® | ŌŚ78g±½ÖŠŗ¬3NAøöĢ¼Ģ¼Ė«¼ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
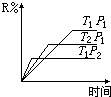 ŌŚĆܱÕČŻĘ÷ÖŠ½ųŠŠĻĀĮŠ·“Ó¦£ŗM£ØĘų£©+N£ØĘų£©?R£ØĘų£©+2S£Ø£æ£©£¬“Ė·“Ó¦·ūŗĻĻĀĆęĶ¼Ļó£¬ĻĀĮŠŠšŹöŹĒÕżČ·µÄŹĒ£Ø””””£©
ŌŚĆܱÕČŻĘ÷ÖŠ½ųŠŠĻĀĮŠ·“Ó¦£ŗM£ØĘų£©+N£ØĘų£©?R£ØĘų£©+2S£Ø£æ£©£¬“Ė·“Ó¦·ūŗĻĻĀĆęĶ¼Ļó£¬ĻĀĮŠŠšŹöŹĒÕżČ·µÄŹĒ£Ø””””£©| A£® | Õż·“Ó¦ĪüČČ£¬SŹĒĘųĢå | B£® | Õż·“Ó¦ĪüČČ£¬SŹĒ¹ĢĢå | ||
| C£® | Õż·“Ó¦·ÅČČ£¬SŹĒĘųĢå | D£® | Õż·“Ó¦·ÅČČ£¬SŹĒ¹ĢĢå»ņŅŗĢå |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ĪĀ¶Č£ØK£© | Ę½ŗāŹ±NH3µÄĪļÖŹµÄĮæ£Ømol£© |
| T1 | 2.4 |
| T2 | 2.0 |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| X | Y | |
| Z | W |
| A£® | ĖÄÖÖ¶ĢÖÜĘŚŌŖĖŲÖŠWµÄŌ×Ó°ė¾¶×īŠ” | |
| B£® | ZŌŖĖŲŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆŹĒµŚČżÖÜĘŚµŚ¢ōA×å | |
| C£® | Ō×Ó×īĶā²ćµē×ÓŹżÓɶąµ½ÉŁµÄĖ³Šņ£ŗY£¾X£¾W£¾Z£¬×īøßÕż¼ŪÓÉøßµ½µĶĖ³Šņ£ŗW£¾Z | |
| D£® | ŌŖĖŲ·Ē½šŹōŠŌÓÉĒæµ½ČõµÄĖ³Šņ£ŗW£¾Z£¬WµÄµ„ÖŹ³£ĪĀĻĀæÉÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
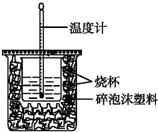 £Ø1£©ŅŃÖŖH+£Øaq£©+OH-£Øaq£©ØTH2O£Øl£©”÷H=-57.3kJ•mol-1£®ÓĆ50mL0.50mol•L-1ŃĪĖįÓė50mL0.55mol•L-1NaOHČÜŅŗŌŚČēĶ¼ĖłŹ¾µÄ×°ÖĆÖŠ½ųŠŠÖŠŗĶ·“Ó¦£®Ķعż²ā¶Ø·“Ó¦¹ż³ĢÖŠĖł·Å³öµÄČČĮææɼĘĖćÖŠŗĶČČ£®»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŅŃÖŖH+£Øaq£©+OH-£Øaq£©ØTH2O£Øl£©”÷H=-57.3kJ•mol-1£®ÓĆ50mL0.50mol•L-1ŃĪĖįÓė50mL0.55mol•L-1NaOHČÜŅŗŌŚČēĶ¼ĖłŹ¾µÄ×°ÖĆÖŠ½ųŠŠÖŠŗĶ·“Ó¦£®Ķعż²ā¶Ø·“Ó¦¹ż³ĢÖŠĖł·Å³öµÄČČĮææɼĘĖćÖŠŗĶČČ£®»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com