
 ��������֮����E����������ȣ�B��C��������Ϊ������D��Eλ��ͬһ���ڣ�D������������������������ڸ������ӵĵ��Ӳ������Իش��������⣺
��������֮����E����������ȣ�B��C��������Ϊ������D��Eλ��ͬһ���ڣ�D������������������������ڸ������ӵĵ��Ӳ������Իش��������⣺ һ�š��������õ�Һ��ȼ�������� ����Ԫ�����ƣ�Ԫ�صĵ��ʣ�
һ�š��������õ�Һ��ȼ�������� ����Ԫ�����ƣ�Ԫ�صĵ��ʣ� ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢ� | B���٢ܢ� | C���ڢۢ� | D���ۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢� | B���ۢ� | C���٢� | D���٢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 ��FΪ��ɢ�ʵĺ��ɫ���塣��ش��������⣺
��FΪ��ɢ�ʵĺ��ɫ���塣��ش��������⣺ ______________��
______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
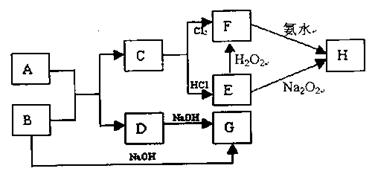
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
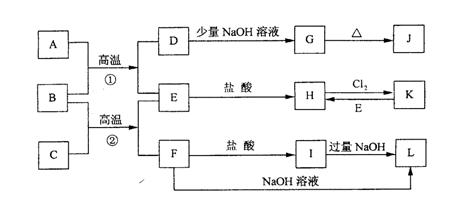
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Ư���е���70%�����ᣬ���̲�������ɫ���壬˵��������л�ԭ�� |
| B����SO2ˮ��Һ�еμ������ữ��BaCl2��Һ���а�ɫ�������ɣ�˵��BaSO4���������� |
| C���ڵ�����Һ�м�������ϡ�����ȣ��ټ���������������ͭ��Һ���ȣ���ɫ������˵������δˮ�� |
| D����2.0mLŨ�Ⱦ�Ϊ0.1mol��L��1��KCl��KI�����Һ�еμ�1~2��0.01mol��L��1 AgNO3��Һ���������ʻ�ɫ��˵��AgCl ��Ksp��AgI ��Ksp�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com