
A����12�֣�CuCl��CuCl2������Ҫ�Ļ���ԭ�ϣ����������������ϡ����������������ȡ�
��֪����CuCl������CuCl2���ʵ��Ļ�ԭ����S02��SnCl2�Ȼ�ԭ�Ƶã�

��CuCl2��Һ���Ҷ���(H2N-CH2-CH2-NH2)���γ������ӣ�
��ش��������⣺
��1����̬Cuԭ�ӵĺ�������Ų�ʽΪ______________��H��N��O����Ԫ�صĵ縺���ɴ�С��˳����_____��
��2��SO2���ӵĿռ乹��Ϊ____����SnCl4��Ϊ�ȵ������һ�����ӵĻ�ѧʽΪ______
��3���Ҷ��������е�ԭ�ӹ�����ӻ�����Ϊ_______���Ҷ��������װ�[N(CH3)3]�����ڰ������Ҷ��������װ��ķе�ߵĶ࣬ԭ����______________________��
��4���������γɵ��������к��еĻ�ѧ��������__________��
a����λ�� b�����Լ� c�����Ӽ� d���Ǽ��Լ�
��5��CuCl�ľ����ṹ����ͼ��ʾ������Clԭ�ӵ���λ��Ϊ_________��

B����12�֣�ij��ѧ�о���ѧϰС��Ϊ̽��ijƷ�ƻ������в�����֬����ĺ���������������ʵ�飺
�����ȡ0.4g��������Ʒ��������������ĵ�ƿ����ͼ���ڣ�����10mL���Ȼ�̼������ҡ��ʹ��ȫ���ܽ⡣���ƿ�м���25.00mL��0.01mol IBr����ˮ������Һ���Ǻ�ƿ�����ڲ�������ƿ��֮��μ�����10%�⻯����Һ��շ�϶������IBr�Ļӷ���ʧ��

������ڰ�������30min������ʱ����ҡ����30min��С�ĵش��������������Ƶ�10%�⻯��10mL������ˮ50mL�Ѳ�������ƿ���ϵ�Һ���ϴ��ƿ�ڡ�
�������ָʾ������0.1mol��L��1�����������Һ�ζ���������ƿ��ֱ���յ㡣
�ⶨ�����з�������ط�Ӧ���£�
�� ��IBr��KI��I2��KBr
��I2��2S2O32����2I����S4O62��
��IBr��KI��I2��KBr
��I2��2S2O32����2I����S4O62��
��ش��������⣺
��1����֪±�ػ�����IBr��������±�ص������ƣ�ʵ����ȷ��ȡIBr��ҺӦ�� ���÷���ʽ��ʾ��ƿ��������ԭ�� ��
��2��������е�ƿ�ڰ�������30min������ʱ����ҡ����ԭ���� ��
��3�������������ָʾ��Ϊ ���ζ��յ������ ��
��4����Ӧ�������Һ�������л������Ȼ�̼������������� ��
��1��1s22s22p63s23p63d104s1��[Ar]3d10 4s1 O >N >H
��2��V�� SO42-��SiO44-��
��3��sp3�ӻ� �Ҷ������Ӽ�����γ���������װ����Ӽ䲻���γ����
��4��abd ��5��4
B����12�֣���1����ʽ�ζ��ܣ�����Һ�ܣ� IBr��H2O��HIO��HBr
��2����ƿ���ڰ������Լ���IBr�Ļӷ�������ҡ�����������ʼ��ַ�Ӧ��
��3��������Һ ��Һ����ɫǡ�ñ�Ϊ��ɫ���Ұ���Ӳ���ɫ��
��4����Һ������ (ÿ��2��,��12��)
��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
A����12�֣�CuCl��CuCl2������Ҫ�Ļ���ԭ�ϣ����������������ϡ����������������ȡ�
��֪����CuCl������CuCl2���ʵ��Ļ�ԭ����S02��SnCl2�Ȼ�ԭ�Ƶã�
��CuCl2��Һ���Ҷ���(H2N-CH2-CH2-NH2)���γ������ӣ�
��ش��������⣺
��1����̬Cuԭ�ӵĺ�������Ų�ʽΪ______________��H��N��O����Ԫ�صĵ縺���ɴ�С��˳����_____��
��2��SO2���ӵĿռ乹��Ϊ____����SnCl4��Ϊ�ȵ������һ�����ӵĻ�ѧʽΪ______
��3���Ҷ��������е�ԭ�ӹ�����ӻ�����Ϊ_______���Ҷ��������װ�[N(CH3)3]�����ڰ������Ҷ��������װ��ķе�ߵĶ࣬ԭ����______________________��
��4���������γɵ��������к��еĻ�ѧ��������__________��
a����λ�� b�����Լ� c�����Ӽ� d���Ǽ��Լ�
��5��CuCl�ľ����ṹ����ͼ��ʾ������Clԭ�ӵ���λ��Ϊ_________��
B����12�֣�ij��ѧ�о���ѧϰС��Ϊ̽��ijƷ�ƻ������в�����֬����ĺ���������������ʵ�飺
�����ȡ0.4g��������Ʒ��������������ĵ�ƿ����ͼ���ڣ�����10mL���Ȼ�̼������ҡ��ʹ��ȫ���ܽ⡣���ƿ�м���25.00mL��0.01mol IBr����ˮ������Һ���Ǻ�ƿ�����ڲ�������ƿ��֮��μ�����10%�⻯����Һ��շ�϶������IBr�Ļӷ���ʧ��
������ڰ�������30min������ʱ����ҡ����30min��С�ĵش��������������Ƶ�10%�⻯��10mL������ˮ50mL�Ѳ�������ƿ���ϵ�Һ���ϴ��ƿ�ڡ�
�������ָʾ������0.1mol��L��1�����������Һ�ζ���������ƿ��ֱ���յ㡣
�ⶨ�����з�������ط�Ӧ���£�
�� ��IBr��KI��I2��KBr ��I2��2S2O32����2I����S4O62��
��ش��������⣺
��1����֪±�ػ�����IBr��������±�ص������ƣ�ʵ����ȷ��ȡIBr��ҺӦ�� ���÷���ʽ��ʾ��ƿ��������ԭ�� ��
��2��������е�ƿ�ڰ�������30min������ʱ����ҡ����ԭ���� ��
��3�������������ָʾ��Ϊ ���ζ��յ������ ��
��4����Ӧ�������Һ�������л������Ȼ�̼������������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��)
�������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݡ���ѡ������һ�����������ⶼ��,��A�����֡�
A��CuCl��CuCl2������Ҫ�Ļ���ԭ�ϣ����������������ϡ����������������ȡ���֪��
��CuCl������CuCl2���ʵ��Ļ�ԭ����SO2��SnCl2�Ȼ�ԭ�Ƶã�
![]()
��CuCl2��Һ���Ҷ���(H2N-CH2-CH2-NH2)���γ������ӣ�
��ش��������⣺
(1)��̬Cuԭ�ӵĺ�������Ų�ʽΪ_________��H��N��O����Ԫ�صĵ縺���ɴ�С��˳����_____��
(2)SO2���ӵĿռ乹��Ϊ____________����SnCl4��Ϊ�ȵ������һ�����ӵĻ�ѧʽΪ_________��
 (3)�Ҷ��������е�ԭ�ӹ�����ӻ�����Ϊ_____________���Ҷ��������װ�[N(CH3)3]�����ڰ������Ҷ��������װ��ķе�ߵĶ࣬ԭ����_______________________________________��
(3)�Ҷ��������е�ԭ�ӹ�����ӻ�����Ϊ_____________���Ҷ��������װ�[N(CH3)3]�����ڰ������Ҷ��������װ��ķе�ߵĶ࣬ԭ����_______________________________________��
(4)�������γɵ��������к��еĻ�ѧ��������__________��(����ĸ)
a����λ�� b�����Լ� c�����Ӽ� d���Ǽ��Լ�
(5)CuCl�ľ����ṹ����ͼ��ʾ������Clԭ�ӵ���λ��Ϊ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݡ���ѡ������һ�����������ⶼ��,��A�����֡�
A��CuCl��CuCl2������Ҫ�Ļ���ԭ�ϣ����������������ϡ����������������ȡ���֪��
��CuCl������CuCl2���ʵ��Ļ�ԭ����SO2��SnCl2�Ȼ�ԭ�Ƶã�
![]()
��CuCl2��Һ���Ҷ���(H2N-CH2-CH2-NH2)���γ������ӣ�
��ش��������⣺
(1)��̬Cuԭ�ӵĺ�������Ų�ʽΪ_________��H��N��O����Ԫ�صĵ縺���ɴ�С��˳����_____��
(2)SO2���ӵĿռ乹��Ϊ____________����SnCl4��Ϊ�ȵ������һ�����ӵĻ�ѧʽΪ_________��
 (3)�Ҷ��������е�ԭ�ӹ�����ӻ�����Ϊ_____________���Ҷ��������װ�[N(CH3)3]�����ڰ������Ҷ��������װ��ķе�ߵĶ࣬ԭ����_______________________________________��
(3)�Ҷ��������е�ԭ�ӹ�����ӻ�����Ϊ_____________���Ҷ��������װ�[N(CH3)3]�����ڰ������Ҷ��������װ��ķе�ߵĶ࣬ԭ����_______________________________________��
(4)�������γɵ��������к��еĻ�ѧ��������__________��(����ĸ)
a����λ�� b�����Լ� c�����Ӽ� d���Ǽ��Լ�
(5)CuCl�ľ����ṹ����ͼ��ʾ������Clԭ�ӵ���λ��Ϊ_________��
B����˾ƥ��(����ˮ����)�dz��õĽ�����ʹҩ�������Ǻϳɰ�˾ƥ�ֵĹ������̡�

��֪���ٷ�Ӧ����ʽΪ


�ڳ��˵�ʵ��װ������ͼ��ʾ��
��ش��й����⣺
(1)����A��������_____________��
(2)�ڲ�����У�����ȴ�ᾧʱδ���ֽᾧ������___________����ʹ����������
(3)ʵ��ʱ��������B��Һ��߶ȿ�ﵽ֧�ܿ�λ��ʱ��Ӧ���еIJ�����____________________________________________��
(4)����C�����������ܵ�λ���Ƿ���ȷ?��_________��(���ȷ������ȷ��)
(5)�ڲ�����У��ñ���NaHC03��Һ���Խ���˾ƥ�ֺв���ȷ��룬�仯ѧԭ����
_________________________________________��Ҫ����Ʒ���Ƿ���ˮ���ᣬ��ʵ�������_____________________________________________________��
(6)�ڲ�����У�����ʹ��Ӧ��ֽ�����_____________Ϊֹ��
(7)��ʵ��õ�2.70g�����İ�˾ƥ�֣����Ʒ�IJ���Ϊ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���ձ�����2010������ڶ��ε��п��� ���ͣ������
�������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݡ���ѡ������һ�����������ⶼ��,��A�����֡�
A��CuCl��CuCl2������Ҫ�Ļ���ԭ�ϣ����������������ϡ����������������ȡ���֪��
��CuCl������CuCl2���ʵ��Ļ�ԭ����S02��SnCl2�Ȼ�ԭ�Ƶã�
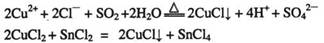
��CuCl2��Һ���Ҷ���(H2N-CH2-CH2-NH2)���γ������ӣ�

��ش��������⣺
(1)��̬Cuԭ�ӵĺ�������Ų�ʽΪ_________��H��N��O����Ԫ�صĵ縺���ɴ�С��˳����_____��
(2)SO2���ӵĿռ乹��Ϊ____________����SnCl4��Ϊ�ȵ������һ�����ӵĻ�ѧʽΪ_________��
(3)�Ҷ��������е�ԭ�ӹ�����ӻ�����Ϊ_____________���Ҷ��������װ�[N(CH3)3]�����ڰ������Ҷ��������װ��ķе�ߵĶ࣬ԭ����_____________________________________________��
 (4)�������γɵ��������к��еĻ�ѧ��������__________��(����ĸ)
(4)�������γɵ��������к��еĻ�ѧ��������__________��(����ĸ)
a����λ�� b�����Լ� c�����Ӽ� d���Ǽ��Լ�
(5)CuCl�ľ����ṹ����ͼ��ʾ������Clԭ�ӵ���λ��Ϊ_________��
B����˾ƥ��(����ˮ����)�dz��õĽ�����ʹҩ�������Ǻϳɰ�˾ƥ�ֵĹ������̡�
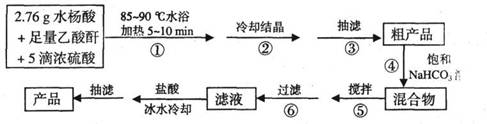
��֪���ٷ�Ӧ����ʽΪ


�ڳ��˵�ʵ��װ������ͼ��ʾ��
��ش��й����⣺
(1)����A��������_____________��
(2)�ڲ�����У�����ȴ�ᾧʱδ���ֽᾧ������___________��
��ʹ����������
(3)ʵ��ʱ��������B��Һ��߶ȿ�ﵽ֧�ܿ�λ��ʱ��Ӧ����
�IJ�����____________________________________________��
(4)����C�����������ܵ�λ���Ƿ���ȷ?��_________��(���ȷ������ȷ��)
(5)�ڲ�����У��ñ���NaHC03��Һ���Խ���˾ƥ�ֺв���ȷ��룬�仯ѧԭ����
_________________________________________��Ҫ����Ʒ���Ƿ���ˮ���ᣬ��ʵ�������_____________________________________________________��
(6)�ڲ�����У�����ʹ��Ӧ��ֽ�����_____________Ϊֹ��
(7)��ʵ��õ�2.70g�����İ�˾ƥ�֣����Ʒ�IJ���Ϊ____________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com