
(15分)某同学为探究元素周期表中元素性质的递变规律,设计了如下系列实验。
Ⅰ.(1)试用实验室提供的下列试剂,设计两个原理不同的简单实验(只要写出实验的方案即可),证明镁元素的金属性比铝元素强。
试剂:镁条、铝条、氯化铝溶液、氯化镁溶液、稀盐酸、氢氧化钠溶液
方案一:_____________________________________________________________;
方案二:_____________________________________________________________。
Ⅱ.利用下图装置可验证同周期元素非金属性的变化规律
(1)仪器A的名称为________,干燥管D的作用为________________。
(2)若要证明非金属性:Cl>S,则A中加浓盐酸,B中加KMnO4(KMnO4与浓盐酸常温下反应生成氯气),C中加___________,观察到C中溶液___________________________的现象,即可证明。反应的离子方程式为________________________________________________________。
从环境保护的观点考虑,此装置缺少尾气处理装置,可用________溶液吸收尾气。
(3)若要证明非金属性:N>C,则在A中加稀硝酸,B中加碳酸钙,C中加澄清石灰水;观察到C中溶液变浑浊的现象,即可证明。该实验原理为______________________________。但有的同学在实验中一切操作正确的情况下没观察到上述现像,试分析可能的原因是___________________________。
Ⅰ(1)方案一:用形状大小相同的铝条和镁条分别一等体积等浓度的稀盐酸反应,观察其反应速率快慢 (2分)
方案二:分别向等体积等浓度的氯化铝溶液、氯化镁溶液中加入氢氧化钠溶液至过量,观察其沉淀及其溶解情况(2分)
Ⅱ(1)分液漏斗;防倒吸(每空1分)
(2)硫化钠溶液(1分)有黄色沉淀生成(1分) S2-+Cl2=2Cl-+S↓(2分) 氢氧化钠溶液(1分)
(3)利用非金属元素最高价氧化物对应水化物酸性强弱来比较元素非金属性强弱 (2分) 硝酸有挥发性,随二氧化碳一起逸出(2分)
解析试题分析:Ⅰ(1)方案一:判断金属性强弱可以依据金属与酸反应的剧烈程度,因此可以设计金属与相同浓度的盐酸生成气体的剧烈程度判断镁和铝金属性强弱;
方案二:根据氢氧化镁、氢氧化铝是否溶于强碱设计,过量碱液可使生成的Al(OH)3溶解,而Mg(OH)2则不溶,具体方法:分别向等体积等浓度的氯化铝溶液、氯化镁溶液中加入氢氧化钠溶液至过量,观察其沉淀及其溶解情况;
Ⅱ(1)根据仪器结构特点可知A为分液漏斗;球形干燥管D能够防止倒吸,可以避免C中液体进入锥形瓶中;
(2)若要证明非金属性:Cl>S,可以利用单质之间的置换反应。B中产生氯气,则在C中加入硫化钠溶液,;氯气与硫化钠分子置换反应生成氯化钠和单质硫,所以观察到C中溶液有黄色沉淀生成的现象,即可证明,反应的离子方程式为S2-+Cl2=2Cl-+S↓;氯气是一种有毒气体,必须进行尾气吸收,氯气能够与氢氧化钠溶液反应,可以使用氢氧化钠溶液吸收多余的氯气;
(5)要证明非金属性是N>C,则可以利用最高价氧化物水化物的酸性强弱来比较,依据较强的酸可以制备较弱的酸可知,如果在A中加稀硝酸,B中加碳酸钙,则B中产生CO2,CO2能使澄清石灰水变浑浊,因此C中加澄清石灰水;观察到C中溶液变浑浊的现象,即可证明。由于硝酸有挥发性,随二氧化碳一起逸出进入澄清的石灰水,因此没观察到上述现像。
考点:考查金属性和非金属性强弱比较的实验方案设计与评价


 阅读快车系列答案
阅读快车系列答案科目:高中化学 来源: 题型:单选题
下列实验装置是探究铜丝与过量浓硫酸的反应,下列叙述正确的是 ( )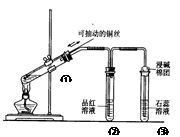
| A.上下移动①中铜丝可控制SO2的量 |
| B.②中品红溶液不褪色 |
| C.③中石蕊溶液变蓝色 |
| D.为确认CuSO4生成,向①中加水,观察溶液颜色 |
查看答案和解析>>
科目:高中化学 来源: 题型:实验题
(20分)工业上常利用含硫废水生产Na2S2O3?5H2O,实验室可用如下装置(略去部分加持仪器)模拟生成过程。
烧瓶C中发生反应如下:
Na2S(aq)+H2O(l)+SO2(g)=Na2SO3(aq)+H2S(aq) (I)
2H2S(aq)+SO2(g)=3S(s)+2H2O(l) (II)
S(s)+Na2SO3(aq) Na2S2O3(aq) (III)
Na2S2O3(aq) (III)
(1)仪器组装完成后,关闭两端活塞,向装置B中的长颈漏斗内注入液体至形成一段液注,若 ,则整个装置气密性良好。装置D的作用是
。装置E中为 溶液。
(2)为提高产品纯度,应使烧瓶C中Na2S和Na2SO3恰好完全反应,则烧瓶C中Na2S和Na2SO3物质的量之比为 。
(3)装置B的作用之一是观察SO2的生成速率,其中的液体最好选择 。
a.蒸馏水 b.饱和Na2SO3溶液
c.饱和NaHSO3溶液 d.饱和NaHCO3溶液
实验中,为使SO2缓慢进入烧瓶C,采用的操作是 。已知反应(III)相对较慢,则烧瓶C中反应达到终点的现象是 。反应后期可用酒精灯适当加热烧瓶A,实验室用酒精灯加热时必须使用石棉网的仪器含有 。
a.烧杯 b.蒸发皿 c.试管 d.锥形瓶
(4)反应终止后,烧瓶C中的溶液经蒸发浓缩即可析出Na2S2O3?5H2O,其中可能含有Na2SO3、Na2SO4等杂质。利用所给试剂设计实验,检测产品中是否存在Na2SO4,简要说明实验操作,现象和结论:
。
已知Na2S2O3?5H2O遇酸易分解:S2O32?+2H+=S↓+SO2↑+H2O
供选择的试剂:稀盐酸、稀硫酸、稀硝酸、BaCl2溶液、AgNO3溶液
查看答案和解析>>
科目:高中化学 来源: 题型:实验题
(15分)实验室从含碘废液(除H2O外,含有CCl4、I2、I-等)中回收碘,其实验过程如下:
(1)向含碘废液中加入稍过量的Na2SO3溶液,将废液中的I2还原为I-,其离子方程式为 ;该操作将I2还原为I-的目的是 。
(2)操作X的名称为 。
(3)氧化时,在三颈烧瓶中将含I-的水溶液用盐酸调至pH约为2,缓慢通入Cl2,在400C左右反应(实验装置如图所示)。实验控制在较低温度下进行的原因是 ;锥形瓶里盛放的溶液为 。
(4)已知:5SO32—+2IO3—+2H+ I2+5SO42—+H2O
I2+5SO42—+H2O
某含碘废水(pH约为8)中一定存在I2,可能存在I-、IO3—中的一种或两种。请补充完整检验含碘废水中是否含有I-、IO3—的实验方案:取适量含碘废水用CCl4多次萃取、分液,直到水层用淀粉溶液检验不出碘单质存在; 。
实验中可供选择的试剂:稀盐酸、淀粉溶液、FeCl3溶液、Na2SO3溶液
查看答案和解析>>
科目:高中化学 来源: 题型:实验题
菠菜营养丰富,长期以来民间流传着“菠菜不能与豆腐同食”的说法。某学校化学兴趣小组的同学拟通过实验探究:菠菜是否含有草酸类物质?草酸又有哪些性质?通过上网查询,获得以下资料:草酸又名乙二酸,其酸性比乙酸稍强,草酸及其盐具有较强的还原性,草酸晶体(H2C2O4·2H2O)的熔点为100.1℃,在175℃时受热分解,草酸钙是难溶于水的白色固体。
他们设计的实验步骤如下:
1.将菠菜在少量开水中煮沸2~3 min,冷却后滤去菠菜,得滤液(含有少量 杂质)。向滤液中加入足量Ca(OH)2溶液,然后再加入足量CH3COOH溶液,观察现象。
杂质)。向滤液中加入足量Ca(OH)2溶液,然后再加入足量CH3COOH溶液,观察现象。
2.用草酸晶体(H2C2O4·2H2O)做以下实验:
请回答以下问题:
(1)步骤1中加入CH3COOH溶液的作用: 。
(2)A处应选择 (填“Ⅰ”或“Ⅱ”),在做实验之前,应先 。
(3)实验2过程中观察到C、E装置中的溶液均变浑浊,且D装置中黑色粉末变为红色,写出A中草酸晶体(H2C2O4·2H2O)发生反应的化学方程式: ,装置B的作用是 。
(4)为使实验结论更加严密和安全,在以上所连接的装置C、D间还需依次添加装有 、 、 (液体试剂)的洗气瓶,此外指出上述装置中的不足之处还有 。
查看答案和解析>>
科目:高中化学 来源: 题型:实验题
目前流行的关于生命起源假设的理论认为,生命起源于约40亿年前的古洋底的热液环境。这种环境系统中普遍存在铁硫簇结构,如Fe2S2、Fe4S4、Fe8S7等,这些铁硫簇结构参与了生命起源的相关反应。某化学兴趣小组在研究某铁硫簇结构的组成时,设计了下列实验。
【实验Ⅰ】 硫的质量确定: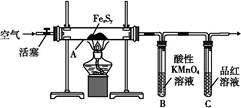
按图连接装置,检查好装置的气密性后,在硬质玻璃管A中放入1.0 g铁硫簇结构(含有部分不反应的杂质),在试管B中加入50 mL 0.1 mol·L-1的酸性KMnO4溶液,在试管C中加入品红溶液。通入空气并加热,发现固体逐渐转变为红棕色。待固体完全转化后将B中溶液转移至250 mL容量瓶,洗涤试管B后定容。取25.00 mL该溶液用0.01 mol·L-1的草酸(H2C2O4)进行测定剩余KMnO4溶液浓度的滴定。记录数据如下:
| 滴定次数 | 待测溶液 体积/mL | 草酸溶液体积/mL | |
| 滴定前刻度 | 滴定后刻度 | ||
| 1 | 25.00 | 1.50 | 23.70 |
| 2 | 25.00 | 1.02 | 26.03 |
| 3 | 25.00 | 0.00 | 24.99 |
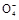 +2H2O+5SO2
+2H2O+5SO2 2Mn2++5S
2Mn2++5S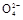 +4H+
+4H+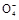 +6H++5H2C2O4
+6H++5H2C2O4 2Mn2++10CO2↑+8H2O
2Mn2++10CO2↑+8H2O| 编号 | 温度/℃ | 酸化的H2C2O4 溶液/mL | KMnO4 溶液/mL | 溶液褪 色时间/s |
| 1 | 25 | 5.0 | 2.0 | 40 |
| 2 | 25 | 5.0(另加少量可溶 于水的MnSO4粉末) | 2.0 | 4 |
| 3 | 60 | 5.0 | 2.0 | 25 |
查看答案和解析>>
科目:高中化学 来源: 题型:实验题
碱式碳酸盐A可用作胃药,其组成可表示为Al2Mg6(OH)x(CO3)y·zH2O。某校化学兴趣小组欲测定其化学式,实验设计如下:
实验I:称取一定质量的A,加热分解至恒重。
实验Ⅱ:称取一定质量的A与足量的酸反应,测量生成CO2气体的质量。
可供选择的仪器和药品如图所示:(酸溶液限选6mol/LHCl或6mol/LH2SO4,其它试剂任选。)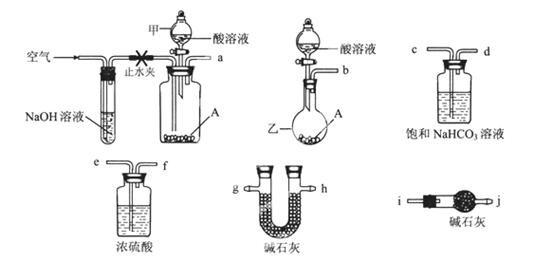
回答下列问题:
(1)仪器乙的名称为________。
(2)请选择必要的装置完成实验II,正确的连接顺序为________ (按气流方向,用接口字母表示);选用的酸溶液是________。
(3)有人提出不采用实验I,可在实验II结束后,在A完全反应后所得溶液中滴加足量的氨水,用无灰滤纸过滤,用蒸馏水洗涤反应容器2?3次,将洗涤液过滤,洗涤沉淀2?3次,将附着沉淀的滤纸放到坩埚中加热分解至恒重。判断沉淀已洗涤干净的方法是_________________,实际上未采用该方案的原因是不符合实验设计的________原则(填字母编号)。
| A.科学性 | B.安全性 | C.可行性 | D.简约性 |
查看答案和解析>>
科目:高中化学 来源: 题型:实验题
(1)某厂利用生产磷铵排放的磷石膏废渣制硫酸联产水泥,硫酸返回用于生产磷铵。其生产流程图如下: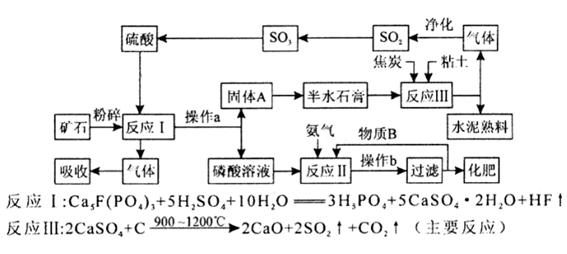
①操作b的名称是浓缩、冷却、 。
②如反应II的产物是两种酸式盐,则物质B中溶质的化学式是 。
③若在实验室中对反应II产生的气体用 吸收。
④该生产流程最大的优点是尽可能地实现原料的循环使用和副产物的综合利用,该生产流程体现的基本思想是 。
(2)为测定同体磷铵化肥中铵态氮的质量分数,实验室用下图所示装置进行实验。实验时,在A中加入mg磷铵样品,关闭止水夹a.打开止水夹b,向A中加入足量的浓NaOH溶液,完全反应后C中浓硫酸增重ng。请回答下列问题:
①试说明检查该装置气密性的操作方法和实验现象 。
②装置B的作用是 ,装置D的作用是 。
③实验过程应该在何时鼓入空气?答: (填“开始前”、“过程中”或“反应后”)
④如某次测定的铵态氮的质量分数明显偏低,则可能的原因是 (填字母)。
| A.磷铵样品与氢氧化钠未充分反应 | B.A和B中残留了一定量的氨气 |
| C.氢氧化钠溶液的浓度太大 | D.鼓气过快 |
查看答案和解析>>
科目:高中化学 来源: 题型:实验题
(14分)正丁醚常用作有机反应的溶剂。实验室制备正丁醚的反应和主要实验装置如下: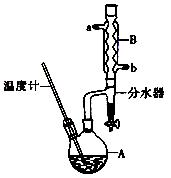
2CH3CH2CH2CH2OH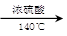 (CH3CH2CH2CH2)2O + H2O
(CH3CH2CH2CH2)2O + H2O
反应物和产物的相关数据如下
| | 相对分子质量 | 沸点/℃ | 密度(g/cm3) | 水中溶解性 |
| 正丁醇 | 74 | 117.2 | 0.819 | 微溶 |
| 正丁醚 | 130 | 142.0 | 0.7704 | 几乎不溶 |
查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com