
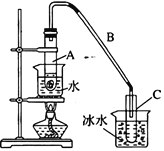
”¾ĢāÄæ”æij»ÆѧŠ”×é²ÉÓĆĄąĖĘÖĘŅŅĖįŅŅõ„µÄ×°ÖĆ£ØČēÓŅĶ¼ĖłŹ¾£©£¬ÓĆ»·¼ŗ“¼Öʱø»·¼ŗĻ©”£
ŅŃÖŖ£ŗ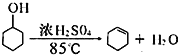
Ļą¶Ō·Ö×ÓÖŹĮæ | ĆܶČ/g cm-3 | ČŪµć/”ę | ·Šµć/”ę | ČܽāŠŌ | |
»·¼ŗ“¼ | 100 | 0.96 | 25 | 161 | ÄÜČÜÓŚĖ® |
»·¼ŗĻ© | 82 | 0.81 | -103 | 83 | ÄŃČÜÓŚĖ® |
£Ø1£©Öʱø“ÖĘ·
½«12.5 mL»·¼ŗ“¼Óė1mLÅØĮņĖį¼ÓČėŹŌ¹ÜAÖŠ£¬Ņ”ŌČŗó·ÅČėĖé“Éʬ£¬»ŗĀż¼ÓČČÖĮ·“Ó¦ĶźČ«£¬ŌŚŹŌ¹ÜCÄŚµĆµ½»·¼ŗĻ©“ÖĘ·”£
¢ŁŌŚŹŌ¹ÜÖŠ»ģŗĻ»·ŅŃ“¼ŗĶÅØĮņĖį²Ł×÷Ź±£¬¼ÓČėŅ©Ę·µÄĻČŗóĖ³ŠņĪŖ_________”£
¢ŚČē¹ū¼ÓČČŅ»¶ĪŹ±¼äŗó·¢ĻÖĶü¼Ē¼ÓĖé“Éʬ£¬Ó¦øĆ²ÉČ”µÄÕżČ·²Ł×÷ŹĒ_________£ØĢī×ÖÄø£©”£
A.Į¢¼“²¹¼Ó B.ĄäČ“ŗó²¹¼Ó C.²»Šč²¹¼Ó D.ÖŲŠĀÅäĮĻ
¢Ū½«ŹŌ¹ÜCÖĆÓŚ±łĖ®ÖŠµÄÄæµÄŹĒ_______________________________”£
£Ø2£©Öʱø¾«Ę·
¢Ł»·¼ŗĻ©“ÖĘ·ÖŠŗ¬ÓŠ»·¼ŗ“¼ŗĶÉŁĮæĖįŠŌŌÓÖŹµČ”£Ļņ“ÖĘ·ÖŠ¼ÓČė±„ŗĶŹ³ŃĪĖ®£¬Õńµ“”¢¾²ÖĆ”¢·Ö²ć£¬»·¼ŗĻ©ŌŚ__________________²ć(Ģī”°ÉĻ”±»ņ”°ĻĀ”±)£¬·ÖŅŗŗóÓĆ__________________£ØĢī×ÖÄø£©Ļ“µÓ”£
a.ĖįŠŌKMnO4ČÜŅŗ b.Ļ”ĮņĖį c.Na2CO3ČÜŅŗ
¢ŚŌŁ½«Ģį“æŗóµÄ»·¼ŗĻ©°“ČēĶ¼ĖłŹ¾×°ÖĆ½ųŠŠÕōĮó”£Ķ¼ÖŠŅĒĘ÷a µÄĆū³ĘŹĒ_______________”£ŹµŃéÖŠĄäČ“Ė®“Ó__________£ØĢī×ÖÄø£©æŚ½ųČė”£ÕōĮóŹ±ŅŖ¼ÓČėÉśŹÆ»Ņ£¬ÄæµÄŹĒ__________________________”£
£Ø3£©ČōŌŚÖʱø“ÖĘ·Ź±»·ŅŃ“¼Ėę²śĘ·Ņ»ĘšÕō³ö£¬ŌņŹµŃéÖʵƵĻ·¼ŗĻ©¾«Ę·ÖŹĮæ__________(Ģī”°øßÓŚ”±”¢”° µĶÓŚ”±)ĄķĀŪ²śĮ攣±¾ŹµŃéĖłµĆµ½µÄ»·ŅŃĻ©ÖŹĮæĪŖ6.25g£¬Ōņ²śĀŹŹĒ___________________”£
”¾“š°ø”æ ĻČ½«»·¼ŗ“¼¼ÓČėŹŌ¹ÜAÖŠ£¬ŌŁ»ŗĀż¼ÓČėÅØĮņĖį B ·ĄÖ¹»·ŅŃĻ©µÄ»Ó·¢£ØĘäĖūŗĻĄķ“š°øŅ²øų·Ö£© ÉĻ c ÕōĮóÉÕĘæ g ĪüŹÕŹ£ÓąµÄĖ® µĶÓŚ 63.5%
”¾½āĪö”æ(1)¢Łøł¾ŻÖʱøŅŅĖįŅŅõ„µÄ²Ł×÷£¬ŌŚŹŌ¹ÜÖŠ»ģŗĻ»·ŅŃ“¼ŗĶÅØĮņĖį²Ł×÷Ź±£¬Ņ©Ę·µÄ¼ÓČė·½·ØĪŖĻČ½«»·¼ŗ“¼¼ÓČėŹŌ¹ÜAÖŠ£¬ŌŁ»ŗĀż¼ÓČėÅØĮņĖį£¬¹Ź“š°øĪŖ£ŗĻČ½«»·¼ŗ“¼¼ÓČėŹŌ¹ÜAÖŠ£¬ŌŁ»ŗĀż¼ÓČėÅØĮņĖį£»
¢ŚČē¹ū¼ÓČČŅ»¶ĪŹ±¼äŗó·¢ĻÖĶü¼Ē¼ÓĖé“Éʬ£¬ŠčŅŖĄäČ“ŗó²¹¼Ó£¬¹ŹŃ”B£»
¢Ū»·ŅŃĻ©µÄČŪµćŗܵĶ£¬·ŠµćŅ²²»øߣ¬ČŻŅ×»Ó·¢£¬½«ŹŌ¹ÜCÖĆÓŚ±łĖ®æÉŅŌ·ĄÖ¹»·ŅŃĻ©µÄ»Ó·¢£¬¹Ź“š°øĪŖ£ŗ·ĄÖ¹»·ŅŃĻ©µÄ»Ó·¢£»
(2)¢Ł»·¼ŗĻ©²»ČÜÓŚĖ®£¬ĒŅĆܶȱČĖ®Š”£¬Õńµ“”¢¾²ÖĆ”¢·Ö²ćŗó»·¼ŗĻ©ŌŚÉĻ²ć£»ÓÉÓŚ·ÖŅŗŗó»·¼ŗĻ©“ÖĘ·ÖŠ»¹ŗ¬ÓŠÉŁĮæµÄĖįŗĶ»·¼ŗ“¼£¬ĮŖĻėÖʱøŅŅĖįŅŅõ„Ģį“æ²śĪļŹ±ÓĆc(Na2CO3ČÜŅŗ)Ļ“µÓæɳżČ„Ėį£¬²»ÄÜÓĆĖįŠŌøßĆĢĖį¼Ų£¬·ńŌņ»įŃõ»Æ»·¼ŗĻ©£¬¹Ź“š°øĪŖ£ŗÉĻ£»c£»
¢ŚĶ¼ÖŠŅĒĘ÷a ŹĒÕōĮóÉÕĘ棬ĪŖĮĖŹ¹ĄäÄżµÄŠ§¹ūøüŗĆ£¬ĄäČ“Ė®“ÓĄäÄż¹ÜµÄĻĀæŚ¼“gæŚ½ųČė£»ÉśŹÆ»ŅÄÜÓėĖ®·“Ӧɜ³ÉĒāŃõ»ÆøĘ£¬³żČ„ĮĖ²ŠĮōµÄĖ®£¬µĆµ½“æ¾»µÄ»·¼ŗĻ©£»¹Ź“š°øĪŖ£ŗÕōĮóÉÕĘ棻g£»ĪüŹÕŹ£ÓąµÄĖ®£»
(3)ČōŌŚÖʱø“ÖĘ·Ź±»·ŅŃ“¼Ėę²śĘ·Ņ»ĘšÕō³ö£¬»·ŅŃ“¼µÄĄūÓĆĀŹĻĀ½µ£¬ŌņŹµŃéÖʵƵĻ·¼ŗĻ©¾«Ę·ÖŹĮæµĶÓŚĄķĀŪ²śĮ攣12.5 mL»·¼ŗ“¼µÄÖŹĮæĪŖ12.5 mL”Į0.96 g cm-3=12g£¬ĄķĀŪÉĻÉś³É»·ŅŃĻ©![]() ”Į82g/mol=9.84g£¬²śĀŹ=
”Į82g/mol=9.84g£¬²śĀŹ=![]() ”Į100%=63.5%£¬¹Ź“š°øĪŖ£ŗµĶÓŚ£»63.5%”£
”Į100%=63.5%£¬¹Ź“š°øĪŖ£ŗµĶÓŚ£»63.5%”£


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠ±ä»ÆÖŠ£¬ŠčŅŖ¼ÓČė»¹Ō¼ĮµÄŹĒ
A.2Cl”Ŗ”śCl2B.Fe3£«”śFe2£«C.NH4+”śNH3D.CO32”ś”śCO2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĖ®µÄµēĄėĘ½ŗāĒśĻßČēÓŅĶ¼ĖłŹ¾£¬ĻĀĮŠĖµ·ØÖŠ£¬ÕżČ·µÄŹĒ
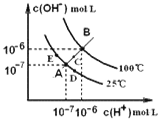
A. Ķ¼ÖŠA”¢B”¢DČżµć“¦KW¼äµÄ¹ŲĻµ£ŗ B>A>D
B. 100”ę£¬ĻņpH=2µÄĻ”ĮņĖįÖŠÖšµĪ¼ÓČėµČĢå»żpH=10µÄĻ”°±Ė®£¬ČÜŅŗÖŠ c(NH4+)£Æc(NH3”¤H2O)¼õŠ”£¬³ä·Ö·“Ó¦ŗó£¬ČÜŅŗµ½“ļBµć
C. ĪĀ¶Č²»±ä£¬ŌŚĖ®ÖŠ¼ÓČėŹŹĮæNH4Cl¹ĢĢ壬æÉ“ÓAµć±ä»Æµ½Cµć
D. ¼ÓČČÅØĖõAµćĖłŹ¾ČÜŅŗ£¬æÉ“ÓAµć±ä»Æµ½Bµć
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠ·ÖĄė·½·ØÓėČܽā¶ČÓŠ¹ŲµÄŹĒ
A.Éż»ŖB.ŻĶČ”C.Äż»ŖD.“ÅĢśĪüŅż
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĶ¼ĖłŹ¾Ōµē³ŲµÄ×Ü·“Ó¦ĪŖCu(s)+2Ag+(aq)![]() Cu2+(aq)+2Ag(s)£¬ĻĀĮŠŠšŹöÕżČ·µÄŹĒ
Cu2+(aq)+2Ag(s)£¬ĻĀĮŠŠšŹöÕżČ·µÄŹĒ
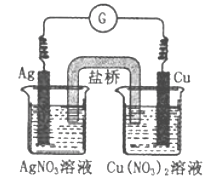
A. µē×Ó“ÓŅųµē¼«¾µ¼ĻßĮ÷ĻņĶµē¼«
B. ¹¤×÷Ņ»¶ĪŹ±¼äŗó£¬ÓŅÉÕ±ÖŠČÜŅŗµÄpH±äŠ”
C. µē³Ų¹¤×÷Ź±£¬Cu2+ĻņĶµē¼«ŅʶÆ
D. ½«AgNO3ČÜŅŗøü»»ĪŖFe(NO3)3ČÜŅŗ£¬µēĮ÷¼ĘÖøÕė·“ĻņĘ«×Ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”潫 100 mL 0.1 mol L-1 FeCl3 ČÜŅŗŗĶ 200 mL 0.3 mol L-1 NaCl ČÜŅŗ»ģŗĻŗó(²»æ¼ĀĒ»ģŗĻŗóČÜŅŗ×ÜĢå»żµÄ±ä»Æ£©£¬Ōņ»ģŗĻŅŗÖŠCl-µÄÅØ¶ČŹĒ
A.0.1 molL-1B.0.3 mol L-1C.0.6 mol L-1D.0.4mol L-1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŹµŃéŹŅÓūÓĆNaOH¹ĢĢåÅäÖĘ1.0 mol”¤L1µÄNaOHČÜŅŗ240 mL£ŗ
(1)ÅäÖĘČÜŅŗŹ±£¬Ņ»°ćæÉŅŌ·ÖĪŖŅŌĻĀ¼øøö²½Öč£ŗ
¢Ł³ĘĮæ””¢Ś¼ĘĖć””¢ŪČܽā””¢Üµ¹×ŖŅ”ŌČ””¢Ż×ŖŅĘ ¢ŽĻ“µÓ””¢ß¶ØČŻ””¢ąĄäČ“
ĘäÕżČ·µÄ²Ł×÷Ė³ŠņĪŖ________________”£±¾ŹµŃé±ŲŠėÓƵ½µÄŅĒĘ÷ÓŠĢģĘ½”¢Ņ©³×”¢²£Į§°ō”¢ÉÕ±”¢½ŗĶ·µĪ¹Ü”¢»¹ÓŠ________”£
(2)ijĶ¬Ń§Óū³ĘĮæŅ»¶ØĮæµÄNaOH¹ĢĢ壬ĖūĻČÓĆĶŠÅĢĢģĘ½³ĘĮæÉÕ±µÄÖŹĮ棬ĢģĘ½Ę½ŗāŗóµÄדĢ¬ČēĶ¼”£ÉÕ±µÄŹµ¼ŹÖŹĮæĪŖ____________ g£¬ŅŖĶź³É±¾ŹµŃéøĆĶ¬Ń§Ó¦³Ę³ö____________ g NaOH”£
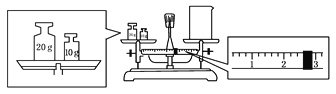
(3)Ź¹ÓĆČŻĮæĘæĒ°±ŲŠė½ųŠŠµÄŅ»²½²Ł×÷ŹĒ________”£
(4)ŌŚÅäÖĘ¹ż³ĢÖŠ£¬ĘäĖū²Ł×÷¶¼ŹĒÕżČ·µÄ£¬ĻĀĮŠ²Ł×÷»įŅżĘšÅضČĘ«øߵďĒ________”£
¢ŁĆ»ÓŠĻ“µÓÉÕ±ŗĶ²£Į§°ō
¢Ś×ŖŅĘČÜŅŗŹ±²»É÷ÓŠÉŁĮæČ÷µ½ČŻĮæĘæĶāĆę
¢ŪČŻĮæĘæ²»øÉŌļ£¬ŗ¬ÓŠÉŁĮæÕōĮóĖ®
¢Ü¶ØČŻŹ±ø©ŹÓæĢ¶ČĻß
¢ŻĪ“ĄäČ“µ½ŹŅĪĀ¾Ķ½«ČÜŅŗ×ŖŅʵ½ČŻĮæĘæ²¢¶ØČŻ
¢Ž¶ØČŻŗóČūÉĻĘæČū·“ø“Ņ”ŌČ£¬¾²ÖĆŗó£¬ŅŗĆęµĶÓŚæĢ¶ČĻߣ¬ŌŁ¼ÓĖ®ÖĮæĢ¶ČĻß
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ”°84Ļū¶¾Ņŗ”±ÄÜÓŠŠ§É±Ćš¼×ŠĶH1N1²”¶¾£¬Ä³Ķ¬Ń§¹ŗĀņĮĖŅ»Ęæ”°ĶžĀ¶Źæ”±ÅĘ”°84Ļū¶¾Ņŗ”±£¬²¢²éŌÄĻą¹Ų׏ĮĻŗĶĻū¶¾Ņŗ°ü×°ĖµĆ÷µĆµ½ČēĻĀŠÅĻ¢£ŗ”°84Ļū¶¾Ņŗ”±£ŗŗ¬25%NaClO”¢1 000 mL”¢ĆܶČ1.192 g”¤cm3£¬Ļ”ŹĶ100±¶(Ģå»ż±Č)ŗóŹ¹ÓĆ”£Ēėøł¾ŻŅŌÉĻŠÅĻ¢ŗĶĻą¹ŲÖŖŹ¶»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©øĆ”°84Ļū¶¾Ņŗ”±µÄĪļÖŹµÄĮæÅضČĪŖ________mol”¤L1”£
£Ø2£©øĆĶ¬Ń§Č”100 mL”°ĶžĀ¶Źæ”±ÅĘ”°84Ļū¶¾Ņŗ”±Ļ”ŹĶŗóÓĆÓŚĻū¶¾£¬Ļ”ŹĶŗóµÄČÜŅŗÖŠc(Na+)=________mol”¤L1”£
£Ø3£©Ņ»Ęæ”°ĶžĀ¶Źæ”±ÅĘ”°84Ļū¶¾Ņŗ”±ÄÜĪüŹÕæÕĘųÖŠ________LµÄCO2(±ź×¼×“æö)¶ų±äÖŹ”£(ŅŃÖŖ£ŗCO2+2NaClO+H2O£½Na2CO3+2HClO)
£Ø4£©øĆĶ¬Ń§²ĪŌÄ”°ĶžĀ¶Źæ”±ÅĘ”°84Ļū¶¾Ņŗ”±µÄÅä·½£¬ÓūÓĆNaClO¹ĢĢåÅäÖĘ480 mLŗ¬25%NaClOµÄĻū¶¾Ņŗ”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ________”£

A£®ČēĶ¼ĖłŹ¾µÄŅĒĘ÷ÖŠ£¬ÓŠĖÄÖÖŹĒ²»ŠčŅŖµÄ£¬»¹ŠčŅ»ÖÖ²£Į§ŅĒĘ÷
B£®ČŻĮæĘæÓĆÕōĮóĖ®Ļ“¾»ŗó£¬Ó¦ŗęøɲÅÄÜÓĆÓŚČÜŅŗÅäÖĘ
C£®ĄūÓĆ¹ŗĀņµÄÉĢĘ·NaClOĄ“ÅäÖĘæÉÄܵ¼ÖĀ½į¹ūĘ«µĶ
D£®ŠčŅŖ³ĘĮæµÄNaClO¹ĢĢåÖŹĮæĪŖ143 g
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŅŃÖŖ£ŗ25”ꏱ£¬0.1mol”¤L-lCH3COOHµÄµēĄė¶Č£ØŅѵēĄėµÄCH3COOH·Ö×ÓŹż/ŌCH3COOH·Ö×Ó×ÜŹż£©Ō¼ĪŖ1%”£øĆĪĀ¶ČĻĀ£¬ÓĆ0.1000mol”¤L-l°±Ė®µĪ¶Ø10.00 mL0.1000mol”¤L-lCH3COOHČÜŅŗ£¬ČÜŅŗµÄpHÓėČÜŅŗµÄµ¼µēÄÜĮ¦£ØI£©µÄ¹ŲĻµČēĶ¼ĖłŹ¾”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ

A. Mµć”śNµć£¬Ė®µÄµēĄė³Ģ¶ČÖš½„Ōö“ó
B. 25”ꏱ£¬CH3COOH µÄµēĄė³£ŹżŌ¼ĪŖ1.0”Į10-2
C. NµćČÜŅŗÖŠ£¬c(CH3COO-) =c(NH4+)=0.05 mol”¤L-l
D. µ±µĪČė20 mL°±Ė®Ź±£¬ČÜŅŗÖŠc(CH3COO-)>c(NH4+)
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com