
����Ŀ������ҩ���ַ������м���M�ĺϳ�·�����£�
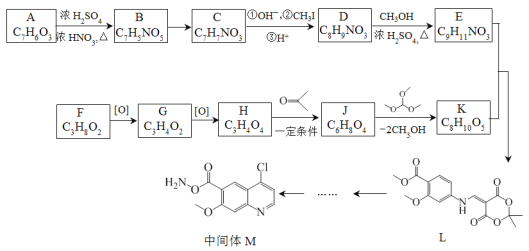
��֪��
i. ![]()
![]()
![]() (R��������)
(R��������)
ii. �л���ṹ���ü���ʽ��ʾ����(CH3O)3CH�ļ���ʽΪ![]()
(1)A�к��б���������ȡ����������λ��A�к��еĹ����������� _______��ѪҺ��A��Ũ�ȹ�����ʹ���ж����ɾ�����עNaHCO3��Һ�ⶾ��A��NaHCO3��Ӧ�Ļ�ѧ����ʽ��_______��
(2)B����C�Ĺ����У�B������_____(���������ԭ��)��Ӧ��
(3)D����E�Ļ�ѧ����ʽ��________��
(4)д����������������D������һ��ͬ���칹��Ľṹ��ʽ________��
�ٷ����к���������ֱ�����ڱ�����
�ں˴Ź�������ͼ��ʾ�����������ֻ�ѧ������ͬ����ԭ��
�۲�����FeCl3��Һ������ɫ��Ӧ
(5)F�ĺ˴Ź�������ͼ��������壬�����֮��Ϊ2:1:1��F����G�Ļ�ѧ����ʽ��_____��
(6)H����J�Ļ�ѧ����ʽ��_______��
(7)��֪E+K��L+CH3OH��K�Ľṹ��ʽ��________��
���𰸡��ǻ����Ȼ� ![]() +NaHCO3��
+NaHCO3��![]() +H2O+CO2�� ��ԭ
+H2O+CO2�� ��ԭ ![]() +CH3OH
+CH3OH![]()
![]() +H2O
+H2O  ��
�� ��
�� ��
�� HOCH2CH2CH2OH+O2
HOCH2CH2CH2OH+O2![]() OHCCH2CHO+2H2O
OHCCH2CHO+2H2O ![]() +
+ ![]()
![]()
 +H2O
+H2O 
��������
�ϳ�E��·��: A����ʽΪC7H6O5�����ݣ�1����Ϣ��A���б���������ȡ������������λ������A�����е�����������ֻ����-OH��-COOH������AΪ![]() ��A��B�DZ����ϵ�������Ӧ������L�Ľṹ��֪��������λ�����Ȼ��Ķ�λ������BΪ��
��A��B�DZ����ϵ�������Ӧ������L�Ľṹ��֪��������λ�����Ȼ��Ķ�λ������BΪ��![]() ���Ա�L��֪��B��C��-NO2�Ļ�ԭ������CΪ:
���Ա�L��֪��B��C��-NO2�Ļ�ԭ������CΪ:![]() ����ӦC��D���Ȱ���Ϣ i.
����ӦC��D���Ȱ���Ϣ i. ![]()
![]()
![]() (�˴�R����Ϊ-CH3)��ʽ��Ӧ���ٽ������ữ���D��
(�˴�R����Ϊ-CH3)��ʽ��Ӧ���ٽ������ữ���D��![]() ��D����״�����������Ӧ��E��
��D����״�����������Ӧ��E��![]() ��
��
�ϳ�K��·�ߣ�F����ʽΪC3H8O2���ѱ��ͣ���������G��ʧ4��H��������һ��������H����2��O�������С�⣨5����Ϣ��֪��F��G��H�ֱ�ΪHOCH2CH2CH2OH��OHCCH2CHO��![]() ������E�ṹ�ɶ�L���������ߴ��и
������E�ṹ�ɶ�L���������ߴ��и ���ٸ���(7)��Ϣ��E+K��L+CH3OH����K�ķ���ʽ��C8H10O5�������Ƴ�K�Ľṹ��ʽ��
���ٸ���(7)��Ϣ��E+K��L+CH3OH����K�ķ���ʽ��C8H10O5�������Ƴ�K�Ľṹ��ʽ�� ������J�ķ���ʽ��K�Ľṹ�ɶ�K���������ߴ��и
������J�ķ���ʽ��K�Ľṹ�ɶ�K���������ߴ��и ���Ӷ��Ƴ�J�ĽṹΪ��
���Ӷ��Ƴ�J�ĽṹΪ�� ��
��
�������Ƶ��Ľ�������ϣ��ɶԸ���С����н��
��1���������Ϸ����Ƶ��Ľ����֪��AΪ![]() ����Ϊ����ǿ����ϵΪ���Ȼ�>̼��>���ǻ�������A��NaHCO3��Ӧ�Ļ�ѧ����ʽ�ǣ�
����Ϊ����ǿ����ϵΪ���Ȼ�>̼��>���ǻ�������A��NaHCO3��Ӧ�Ļ�ѧ����ʽ�ǣ�![]() +NaHCO3��
+NaHCO3��![]() +H2O+CO2�����ʴ�Ϊ���ǻ����Ȼ�
+H2O+CO2�����ʴ�Ϊ���ǻ����Ȼ� ![]() +NaHCO3��
+NaHCO3��![]() +H2O+CO2��
+H2O+CO2��
��2��B����C�Ĺ����У�Bʧȥ2��O������2��H������ʧ���ǻ�ԭ��Ӧ���ʴ�Ϊ����ԭ
��3���������Ϸ����Ƶ��Ľ����֪�� DΪ![]() ��EΪ
��EΪ![]() ����ӦΪ��
����ӦΪ��![]() +CH3OH
+CH3OH![]()
![]() +H2O���ʴ�Ϊ��
+H2O���ʴ�Ϊ��![]() +CH3OH
+CH3OH![]()
![]() +H2O
+H2O
��4���������ٷ����к���������ֱ�����ڱ����ϣ����D�Ľṹ![]() ��֪�������ϵ�ȡ������һ�������Ͷȣ������������Ͷ�Ϊ1����������ȡ���������ͣ�
��֪�������ϵ�ȡ������һ�������Ͷȣ������������Ͷ�Ϊ1����������ȡ���������ͣ�
�ٸ��������ڣ��˴Ź�������ͼ��ʾ�����������ֻ�ѧ������ͬ����ԭ�ӣ�
�ۣ͢�������FeCl3��Һ������ɫ��Ӧ�������Ƴ�����������D��ͬ���칹��Ľṹ��ʽΪ�� ��
�� ��
�� ��
�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
�� ��
��
(5) �������Ϸ����Ƶ��Ľ����֪��F��G�ֱ�ΪHOCH2CH2CH2OH��OHCCH2CHO����F����G�Ļ�ѧ����ʽ�ǣ�HOCH2CH2CH2OH+O2![]() OHCCH2CHO+2H2O���ʴ�Ϊ��HOCH2CH2CH2OH+O2
OHCCH2CHO+2H2O���ʴ�Ϊ��HOCH2CH2CH2OH+O2![]() OHCCH2CHO+2H2O
OHCCH2CHO+2H2O
(6) �������Ϸ����Ƶ��Ľ����֪��HΪ![]() ��JΪ
��JΪ  ��H����J�Ļ�ѧ����ʽ�ǣ�
��H����J�Ļ�ѧ����ʽ�ǣ�![]() +
+ ![]()
![]()
 +H2O���ʴ�Ϊ��
+H2O���ʴ�Ϊ��![]() +
+ ![]()
![]()
 +H2O
+H2O
(7) �������Ϸ����Ƶ��Ľ����֪��K�Ľṹ��ʽ�ǣ� ���ʴ�Ϊ��
���ʴ�Ϊ��


 �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶��£���3 mol A�����1 mol B����ͨ��һ�ݻ��̶�Ϊ2 L���ܱ������У��������·�Ӧ��3A(g)��B(g)![]() xC(g)������д���пհף�
xC(g)������д���пհף�
(1)��Ӧ1 minʱ���ʣ��1.8 mol A��C��Ũ��Ϊ0.4 mol/L����1 min�ڣ�B��ƽ����Ӧ����Ϊ___________��xΪ____________��
(2)����Ӧ��2 min�ﵽƽ�⣬ƽ��ʱC��Ũ��______________0.8 mol/L����������������С����������������
(3)�ܼӿ췴Ӧ���ʵĴ�ʩ��____________��
�������¶�
������������䣬�����������Ar
������ѹǿ���䣬�����������Ar
��ʹ�ô���
�ݽ����������Сһ��
(4)�ܹ�˵���÷�Ӧ�ﵽƽ��ı�־��____________��
A�������ڻ��������ܶȱ��ֲ���
B�������ڻ�������ѹǿ���ֲ���
C��A��B��Ũ��֮��Ϊ3��1
D����λʱ���ڶϿ�3n mol A-A����ͬʱ����n mol B-B
E��v(A)=3v(B)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ij��Ӧ�ĸ�����Ũ���������£�
����������������aA(g)��bB(g)![]() 2C(g)
2C(g)
��ʼŨ��(mol��L��1) 3.0 1.0 0
2 sĩŨ��(mol��L��1) 1.8 0.6 0.8
�ݴ˿������������Ӧ��ѧ����ʽ�У������ʵĻ�ѧ������֮����(����)
A. 9��3��4B. 3��1��2C. 2��1��3D. 3��2��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ü״���ˮ��������Ϊȼ�ϵ���ṩ�������״���ˮ������������Ҫ��Ӧ�ǣ���Ӧi��CH3OH(g)+H2O(g)![]() CO2(g)+3H2(g) ��H1= +49 kJ/mol��ͬʱ�����ڸ���Ӧ����Ӧii��CH3OH(g)
CO2(g)+3H2(g) ��H1= +49 kJ/mol��ͬʱ�����ڸ���Ӧ����Ӧii��CH3OH(g)![]() CO(g)+2H2(g) ��H2= +91 kJ/mol
CO(g)+2H2(g) ��H2= +91 kJ/mol
(1)��Ӧi��ƽ�ⳣ��K�ı���ʽ��_______��
(2)Ϊ̽�������Է�Ӧiƽ���Ӱ�죬X�� Y(Y1��Y2)�ɷֱ����ѹǿ���¶ȡ���ͼ��ʾYһ��ʱ����Ӧi��H2O(g)��ƽ��ת������X�ı仯��ϵ��

�� X��������������_______��
�� �ж�Y1_______Y2(�>����<��)��������_______��
(3)CO��ʹ��Ӧi�Ĵ����ж����о��¶Ⱥ�Ͷ�ϱȶԼ״�ת���ʼ���������CO���ʵ���������Ӱ�죬�����ͼ��ʾ��

��ѡ��250�桢ˮ/�״�Ͷ�ϱ�Ϊ2��Ϊ����������з�Ӧ��ԭ����_______��
��250��ʱCO���ʵ�������ʼ�ո���200��ʱCO���ʵ���������ԭ�������_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A(C2H4)�ǻ������л�����ԭ�ϡ���A�ͳ������л���ɺϳ�һ���������Ϻ�һ����ȩ�����ϣ�����ϳ�·����ͼ��ʾ�����ַ�Ӧ������ȥ����

��֪��

�ش��������⣺
��1��B�ķ���ʽ��___________��C�к��еĹ����������� ____________��
��2����DΪ��ȡ�������廯��������������Ʒ�Ӧ��ÿ��D������ֻ����1����ԭ�ӣ�D����Ԫ�ص���������ԼΪ13.1%����D�Ľṹ��ʽΪ___________���ķ�Ӧ������________________��
��3���ݱ�������Ӧ�����������£���NaHSO4��H2OΪ�������У���д���˷�Ӧ�Ļ�ѧ����ʽ��____________________________________��
��4����д���������������ı���ȩ������ͬ���칹��Ľṹ��ʽ��___________________��
i .���б�����![]() �ṹ
�ṹ
ii.�˴Ź���������4��壬�ҷ����֮��Ϊ3��2��2��1
��5����������EΪ�����ѵ�ͬϵ�����Է��������ȱ����Ѵ�14������ʹFeCl3��Һ��ɫ��E������ͬ���칹�干��(�����������칹)________________�֡�
��6������ �ĺϳ�·�ߣ�д����2-�ȱ���ͱ�Ҫ�����Լ��Ʊ�
�ĺϳ�·�ߣ�д����2-�ȱ���ͱ�Ҫ�����Լ��Ʊ� �ĺϳ�����ͼ��_______________________________________
�ĺϳ�����ͼ��_______________________________________
�ϳ�����ͼʾ�����£�CH2 = CH2![]() CH3CH2Br
CH3CH2Br![]() CH3CH2OH
CH3CH2OH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������G��һ��ҩ��ϳɵ��м��壬G��һ�ֺϳ�·�����£�
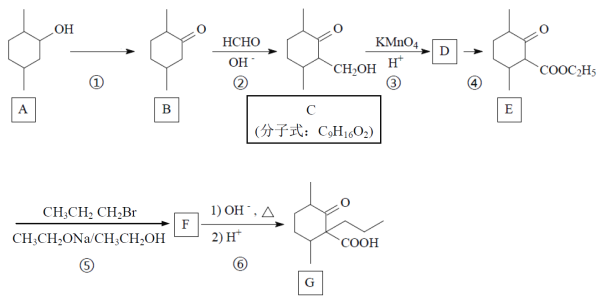
(1)д��A�й����ŵĵ���ʽ��_____________��
(2)д����Ӧ���ͣ�B��C___________��Ӧ��C��D__________��Ӧ��
(3) A��B���跴Ӧ�Լ��ͷ�Ӧ����Ϊ_______________________________��
(4) д��C�ķ�����������ͬ���칹��Ľṹ��ʽ��_________________________��(��д��3��)
����ˮ�⣻���ܷ���������Ӧ������Ԫ���ṹ���һ���ֻ��һ��̼ԭ������ȡ������
(5)д��F�Ľṹ��ʽ_______________________��
(6)����ѧ����֪ʶ��д���ɼױ�(![]() )��
)��![]() Ϊԭ���Ʊ�
Ϊԭ���Ʊ� �ĺϳ�·�ߡ�(���Լ�����)_____________________��
�ĺϳ�·�ߡ�(���Լ�����)_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������л����ʵ�����Ʊ����ռ�װ�ò���ȷ���ǣ� ��
A.���屽
B.ȡ��ϩ
C.��������
D.��ȡ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ��W��X��Y��Z��ԭ�������������ӣ�����ЩԪ����ɵij������ʵ�ת����ϵ����ͼ������a��b��d��gΪ��������aΪ����ɫ������c��Z�ĵ������õ������ˮ������Ӧ���Ҹõ��ʿ�������������fΪ���嵥�ʡ������й�˵����ȷ����

A. �����ӵİ뾶:Y>Z>X B. Ԫ�صķǽ�����:W>X
C. ����������Ӧˮ����ļ���:Y>Z D. X��Y����Ԫ����ɵĻ�����ֻ�����Ӽ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����жԷ��ӵ����ʵĽ�����,����ȷ����

A. ˮ���ȶ�(1000�����ϲŻᲿ�ַֽ�),����ˮ�к��д��������
B. [Cu(NH3)4]SO4��(NH4)2SO4���������ж�����λ��,���Զ��������
C. �����������Ȼ�̼,����������ˮ��������������ԭ������
D. ����ͼ֪����:H3PO4>HClO,��ΪH3PO4�ķ��ǻ���ԭ�������ڴ�����ķ��ǻ���ԭ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com