
| A��KCl | B��KNO3 | C��Na2S | D��CuO |
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ������ | Ba2+ NH4+ Fe3+ Al3+ Fe2+ |
| ������ | OH- CO32- Cl- SO32- SO42- |
 ���������⡣
���������⡣ �����ܽ���ˮ�Ƶã�����εĻ�ѧʽΪ ��
�����ܽ���ˮ�Ƶã�����εĻ�ѧʽΪ �� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
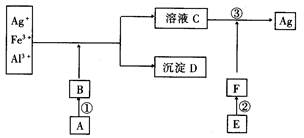
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��pH=1����Һ�У�Fe2+��Al3+��K+��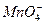 |
B��ij��ɫ��Һ�У�K+��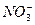 ��Cl-�� ��Cl-��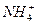 |
C�����д���OH-����Һ�У�Na+��Cu2+��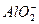 �� ��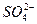 |
D����������ˮ�������c(H+)=10-10 mol��L-1����Һ�У�Na+��Cl-��F-��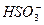 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 �ŷŵĹ�
�ŷŵĹ� ҵ��ˮ�У�����K����Ag����Fe3����C1����OH����NO
ҵ��ˮ�У�����K����Ag����Fe3����C1����OH����NO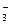 �������ӡ�
�������ӡ� ����д����Ԫ�ط��ţ���
����д����Ԫ�ط��ţ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
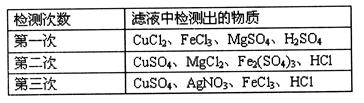
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢ� | B���٢ۢ� | C���ܢݢ� | D���ڢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��
�� �鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com