


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| ||
. |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����һ��ѧ��ͨ�ø��ư���ƥ��ABC���������� ���ͣ�058
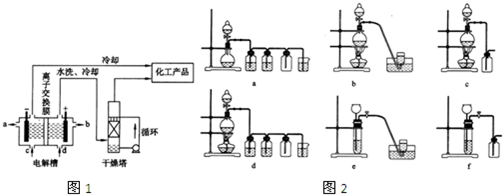
��ͼ��ʵ������ȡ��ˮ�����﴿��������ʵ��װ�ã�

�Իش����и��ʣ�
(1)����Ũ���������������Ӧ����ȡ��������������ʵ�鲽�裬ֱ����Ӧ��ʼ���У�
�ٰ�ͼʾ������װ�����Ӻã����Ҽ�������ԣ�
______________________________________________________________
______________________________________________________________
______________________________________________________________
(2)д��aװ���з�����Ӧ�Ļ�ѧ����ʽ���������ת�Ƶ������
______________________________________________________________
(3)����ȡ��ƿ��ˮ������ƿ��________��������________��
(4)dװ����ʢ�ŵ���________������������________��
(5)eװ�������ռ����������ռ���������________(��л�)�Ȼ��⣬��˵�����ɣ�________��
(6)fװ����ʢ�ŵ���________��Һ������������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������У������һģ���Ի�ѧ�Ծ� ���ͣ�ʵ����
��15�֣�����������ι�ҵ����Ҫ��Ʒ֮һ����ͨ����Ӧ��NaC1O3+KC1
KC1O3��+NaC1��ȡ��
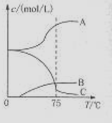
��1��ʵ������ȡ�����ƿ�ͨ����Ӧ��3C12+6NaOH 5NaC1+NaC1O3+3H2O�����ڡ�5���NaOH��Һ��ͨ������C12��ƽ�ⳣ��K=1.09��1012������ʱC12������������Ҫ�� ������Һ���ȣ���Һ����Ҫ����Ũ�����¶ȵı仯����ͼ��ʾ��ͼ��A��B��C���α�ʾ�������� ��
5NaC1+NaC1O3+3H2O�����ڡ�5���NaOH��Һ��ͨ������C12��ƽ�ⳣ��K=1.09��1012������ʱC12������������Ҫ�� ������Һ���ȣ���Һ����Ҫ����Ũ�����¶ȵı仯����ͼ��ʾ��ͼ��A��B��C���α�ʾ�������� ��
��2����ҵ����ȡ�����Ʋ������ȵ�ʯ����ͨ��������Ȼ
��ᾧ��ȥ�Ȼ��ƺ��ټ���һ�����Σ����ʵ�������
��
��3��������ŷ���������������ö���������ˮ������
��Ĥ��ⷨ��ã������������漰����Ҫ�Ļ�ѧ��Ӧʽ���£�
�ܷ�Ӧʽ��NaC1+3H2O NaC1O3+3H2��
NaC1O3+3H2��
������2C1����2e��
C12��������2H2O+2e��
H2��+2OH��
Һ�෴Ӧ��C12+H2O HC1O+H++C1��
HC1O
HC1O+H++C1��
HC1O H++C1O��
H++C1O��
2HC1O+CO�� C1O3��+2C1��+2H+
��
����ʳ��ˮʱ��Ҫ��ȥ���е�Ca2+��Mg2+��SO42�����õ�������Һ�����μ���Ļ�ѧ�Լ�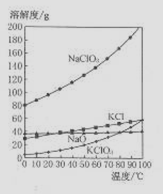
�� �� �� �� �����ˣ���Һ���ټ���������ϡ���ᣬ��һ��������ˮ�پ����ӽ���������Ĥ�����õ�����������ˮ��
�ڵ��ʱ��������ʳ��ˮ�м���Na2Cr2O2����Ŀ���Ƿ�ֹ �������ӷ��ţ����������������Ϸŵ硣
��4����NaC1O2��KC1�Ļ����Һ��NaC1O3��KC1�����������ֱ�Ϊ0.290��0.203��������ʵ��ܽ����������ͼ�����ӻ����Һ�л�ý϶�KC1O3�����ʵ���������
Ϊ ����������ƣ������
��5����Ʒ��C1O3���ĺ������õζ������вⶨ��ʵ�鲽��
���£�
����1��ȷ��ȡ��Ʒag��Լ2.20g�������ܽ⡢���ݵȲ���ȷ����1000mL��Һ��
����2������������ƿ��ȡ��10.00mL����ƿ�У�ȷ����25mL1000mol/L(NH4)2Fe��SO4��2����Һ��������������75mL�����������ɵĻ��ᣬ����10min��
����3��������ƿ�м���100mL����ˮ��ij��������ԭ��Ӧָʾ������0.200mol/LK2Cr2O2����Һ�ζ����յ㡣
����4�� ��
����5�����ݴ�������㡣
�ٲ���2������10min��Ŀ���� ��
�ڲ���3��K2Cr2O2����ҺӦʢ���� �У����������ƣ���
��Ϊ��ȷ����Ʒ��C1O2������������������4�IJ��������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��15�֣�����������ι�ҵ����Ҫ��Ʒ֮һ����ͨ����Ӧ��NaC1O3+KC1 KC1O3��+NaC1��ȡ��
��1��ʵ������ȡ�����ƿ�ͨ����Ӧ��3C12+6NaOH![]() 5NaC1+NaC1O3+3H2O�����ڡ�5���NaOH��Һ��ͨ������C12��ƽ�ⳣ��K=1.09��1012������ʱC12������������Ҫ�� ������Һ���ȣ���Һ����Ҫ����Ũ�����¶ȵı仯����ͼ��ʾ��ͼ��A��B��C���α�ʾ�������� ��
5NaC1+NaC1O3+3H2O�����ڡ�5���NaOH��Һ��ͨ������C12��ƽ�ⳣ��K=1.09��1012������ʱC12������������Ҫ�� ������Һ���ȣ���Һ����Ҫ����Ũ�����¶ȵı仯����ͼ��ʾ��ͼ��A��B��C���α�ʾ�������� ��
��2����ҵ����ȡ�����Ʋ������ȵ�ʯ����ͨ��������Ȼ
��ᾧ��ȥ�Ȼ��ƺ��ټ���һ�����Σ����ʵ�������
��
��3��������ŷ���������������ö���������ˮ������
��Ĥ��ⷨ��ã������������漰����Ҫ�Ļ�ѧ��Ӧʽ���£�
�ܷ�Ӧʽ��NaC1+3H2O![]() NaC1O3+3H2��
NaC1O3+3H2��
������2C1����2e�� C12��������2H2O+2e�� H2��+2OH��
Һ�෴Ӧ��C12+H2O![]() HC1O+H++C1�� HC1O
HC1O+H++C1�� HC1O![]() H++C1O��
H++C1O��
2HC1O+CO�� C1O3��+2C1��+2H+
�� ����ʳ��ˮʱ��Ҫ��ȥ���е�Ca2+��Mg2+��SO42�����õ�������Һ�����μ���Ļ�ѧ�Լ�
�� �� �� �� �����ˣ���Һ���ټ���������ϡ���ᣬ��һ��������ˮ�پ����ӽ���������Ĥ�����õ�����������ˮ��
�ڵ��ʱ��������ʳ��ˮ�м���Na2Cr2O2����Ŀ���Ƿ�ֹ �������ӷ��ţ����������������Ϸŵ硣
��4����NaC1O2��KC1�Ļ����Һ��NaC1O3��KC1�����������ֱ�Ϊ0.290��0.203��������ʵ��ܽ����������ͼ�����ӻ����Һ�л�ý϶�KC1O3�����ʵ���������
Ϊ ����������ƣ������
��5����Ʒ��C1O3���ĺ������õζ������вⶨ��ʵ�鲽��
���£�
����1��ȷ��ȡ��Ʒag��Լ2.20g�������ܽ⡢���ݵȲ���ȷ����1000mL��Һ��
����2������������ƿ��ȡ��10.00mL����ƿ�У�ȷ����25mL1000mol/L(NH4)2Fe��SO4��2����Һ��������������75mL�����������ɵĻ��ᣬ����10min��
����3��������ƿ�м���100mL����ˮ��ij��������ԭ��Ӧָʾ������0.200mol/LK2Cr2O2����Һ�ζ����յ㡣
����4�� ��
����5�����ݴ�������㡣
�ٲ���2������10min��Ŀ���� ��
�ڲ���3��K2Cr2O2����ҺӦʢ���� �У����������ƣ���
��Ϊ��ȷ����Ʒ��C1O2������������������4�IJ��������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ������У������һģ���Ի�ѧ�Ծ� ���ͣ�ʵ����
��15�֣�����������ι�ҵ����Ҫ��Ʒ֮һ����ͨ����Ӧ��NaC1O3+KC1 KC1O3��+NaC1��ȡ��
��1��ʵ������ȡ�����ƿ�ͨ����Ӧ��3C12+6NaOH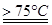 5NaC1+NaC1O3+3H2O�����ڡ�5���NaOH��Һ��ͨ������C12��ƽ�ⳣ��K=1.09��1012������ʱC12������������Ҫ�� ������Һ���ȣ���Һ����Ҫ����Ũ�����¶ȵı仯����ͼ��ʾ��ͼ��A��B��C���α�ʾ�������� ��
5NaC1+NaC1O3+3H2O�����ڡ�5���NaOH��Һ��ͨ������C12��ƽ�ⳣ��K=1.09��1012������ʱC12������������Ҫ�� ������Һ���ȣ���Һ����Ҫ����Ũ�����¶ȵı仯����ͼ��ʾ��ͼ��A��B��C���α�ʾ�������� ��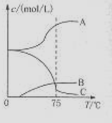
��2����ҵ����ȡ�����Ʋ������ȵ�ʯ����ͨ��������Ȼ
��ᾧ��ȥ�Ȼ��ƺ��ټ���һ�����Σ����ʵ�������
��
��3��������ŷ���������������ö���������ˮ������
��Ĥ��ⷨ��ã������������漰����Ҫ�Ļ�ѧ��Ӧʽ���£�
�ܷ�Ӧʽ��NaC1+3H2O NaC1O3+3H2��
NaC1O3+3H2��
������2C1����2e�� C12��������2H2O+2e�� H2��+2OH��
Һ�෴Ӧ��C12+H2O HC1O+H++C1�� HC1O
HC1O+H++C1�� HC1O H++C1O��
H++C1O��
2HC1O+CO�� C1O3��+2C1��+2H+
����ʳ��ˮʱ��Ҫ��ȥ���е�Ca2+��Mg2+��SO42�����õ�������Һ�����μ���Ļ�ѧ�Լ�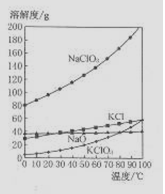
�� �� �� �����ˣ���Һ���ټ���������ϡ���ᣬ��һ��������ˮ�پ����ӽ���������Ĥ�����õ�����������ˮ��
�ڵ��ʱ��������ʳ��ˮ�м���Na2Cr2O7����Ŀ���Ƿ�ֹ �������ӷ��ţ����������������Ϸŵ硣
��4����NaC1O3��KC1�Ļ����Һ��NaC1O3��KC1�����������ֱ�Ϊ0.290��0.203��������ʵ��ܽ����������ͼ�����ӻ����Һ�л�ý϶�KC1O3�����ʵ���������
Ϊ ����������ƣ������
��5����Ʒ��C1O3���ĺ������õζ������вⶨ��ʵ�鲽��
���£�
����1��ȷ��ȡ��Ʒag��Լ2.20g�������ܽ⡢���ݵȲ���ȷ����1000mL��Һ��
����2������������ƿ��ȡ��10.00mL����ƿ�У�ȷ����25mL1000mol/L(NH4)2Fe��SO4��2����Һ��������������75mL�����������ɵĻ��ᣬ����10min��
����3��������ƿ�м���100mL����ˮ��ij��������ԭ��Ӧָʾ������0.200mol/LK2Cr2O7����Һ�ζ����յ㡣
����4�� ��
����5�����ݴ�������㡣
�ٲ���2������10min��Ŀ���� ��
�ڲ���3��K2Cr2O2����ҺӦʢ���� �У����������ƣ���
��Ϊ��ȷ����Ʒ��C1O3������������������4�IJ��������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com