
| ||
| ||
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��

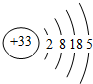
 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ƺ��� | B�������� |
| C��ͭ���� | D�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
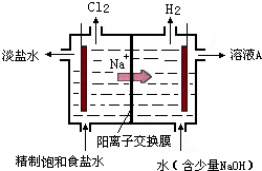
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����⾫��ͭʱ��ͬһʱ���������ܽ�Ĵ�ͭ������������������ͭ�������� |
| B���ڶƼ��ϵ��п��������п��������Ҳ�����ö��Բ��������� |
| C�����ε�س������ǻ�ѧ��ת��Ϊ���ܵĹ��� |
| D�����ε�س��ʱ������ϱ��С�+���ĵ缫Ӧ����ӵ�Դ�ĸ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
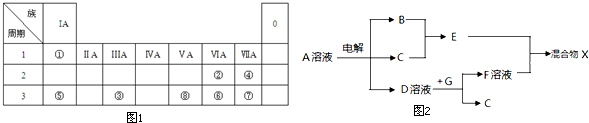
| ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | C | N | O | Ne | ||||
| 3 | Na | Mg | Al | Si | S | Cl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
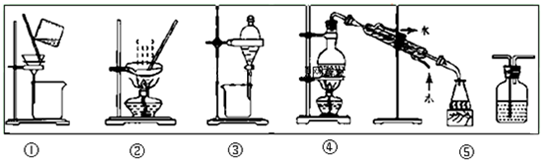
| A�������ᴿ��ѡ�ٺ͢� |
| B����CC14��ȡ��ˮ�еĵ⣬ѡ�� |
| C������Na2CO3��Һ��CH3COOC2H5��ѡ�� |
| D����FeC12��Һ����C12ѡ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
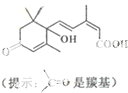 �������˻��ڼ�Դ��������ʻ�ʩ����S-�տ����Ƽ����Ա����ʻ�ʢ����S-�տ��صķ��ӽṹ��ͼ�����й��ڸ÷���˵����ȷ���ǣ�������
�������˻��ڼ�Դ��������ʻ�ʩ����S-�տ����Ƽ����Ա����ʻ�ʢ����S-�տ��صķ��ӽṹ��ͼ�����й��ڸ÷���˵����ȷ���ǣ�������| A��1 mol S-�տ��صķ�������2molNaOH��Ӧ |
| B���ܷ����ӳɡ�ȡ������ȥ��Ӧ |
| C��S-�տ��صķ���ʽΪC14H19O4 |
| D������̼̼˫�����������ǻ����ʻ����Ȼ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���� | B����ϩ | C���Ҵ� | D���Ȼ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com