
ij��ѧ��ȤС��Ϊ��̽��п��Ũ���ᷴӦ��������ijɷ���������ʵ�飺
��50gп����50mLŨH2SO4�ڼ��������³�ַ�Ӧ��п����ʣ�࣬�ռ���һ���������
�壬���������������ɱ�״��Ϊ11.2L��
��1����ѧ��ȤС�����Ƶõ�����X�л��е���Ҫ�������������__________���������ֽ������Ҫԭ���ǣ������ӷ���ʽ��ʾ��_________________ ��
��2��ʵ����֤��Ϊ�˼�����Ҫ��������ijɷ֣���ѧ��ȤС���ͬѧ���������ʵ�飬������Xȡ������̽����
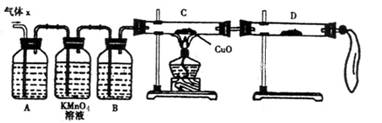
��A�м�����Լ�������__________��B�м�����Լ�������_________��
�ڹ۲쵽C�е�ʵ��������____________________��D�����ѡ����Լ���________��
��3�����۷���������С��ͬѧ�ռ������������Ϊ25.8g������Ũ��������ʵ���Ũ��Ϊ18.0mol/L����ͨ������ȷ������X�и��ɷ����ʵ����ֱ�Ϊ_____________��
 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��8�֣�ij��ѧ��ȤС��Ϊ��̽��п��Ũ���ᷴӦ��������ijɷ���������ʵ�飺
��50gп����50mLŨH2SO4�ڼ��������³�ַ�Ӧ��п����ʣ�࣬�ռ���һ���������
�壬���������������ɱ�״��Ϊ11.2L��
��1����ѧ��ȤС�����Ƶõ�����X�л��е���Ҫ�������������__________���������ֽ������Ҫԭ���ǣ������ӷ���ʽ��ʾ��_________________ ��
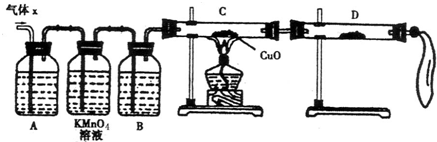
��2��ʵ����֤��Ϊ�˼�����Ҫ��������ijɷ֣���ѧ��ȤС���ͬѧ���������ʵ�飬������Xȡ������̽����
��A�м�����Լ�������__________��B�м�����Լ�������_________��
�ڹ۲쵽C�е�ʵ��������____________________��D�����ѡ����Լ���________��
��3�����۷���������С��ͬѧ�ռ������������Ϊ25.8g������Ũ��������ʵ���Ũ��Ϊ18.0mol/L����ͨ������ȷ������X�и��ɷ����ʵ����ֱ�Ϊ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꺣��ʡ�����и����߿����в��Եػ�ѧ�Ծ� ���ͣ�ʵ����
��11�֣�ij��ѧ��ȤС��Ϊ��̽��п�����ᷴӦ�IJ��ѡ����12mol/L������50ml�������п�ڼ��������·�Ӧ��������Ļ�ԭ�������ȷ�����顣
��1��������衣������ѧ��֪ʶ�����Ƕ�����Ļ�ԭ������������ּ��裺
����1��_________________________________��
����2��_________________________________��
����3����SO2��H2 �������塣
��2�����ʵ�鷽��֤�����衣С��Ϊ��֤������3��ѡ������ͼ��ʾ��������ҩƷ���������������ҵķ��������Ľӿ�˳��Ϊa��
��������ҩƷ���ظ�ʹ�ã���
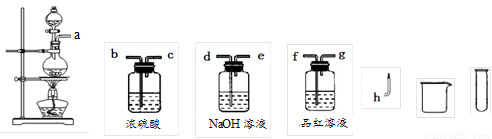
��3��ʵ����̡����ݣ�2���е�ʵ�鷽������ʵ�顣��������������������Ҫ֤����H2������Ӧ���õ�ʵ�����������___________________________________________��
��4��ʵ����ۡ�ͨ������ʵ�飬֤��ȷ��SO2��H2 �����������������������ϸ�������Ľ��ͣ�_______________________________________________________��
��5����Ӧֹͣ����ƿ�е�Һ����ˣ���Һ��ˮϡ�ͣ�����������BaCl2��Һ����ַ�Ӧ����ˣ��õ�81.55g���������ڸ�ʵ�������ɵ�SO2��H2�������Ϊ__________��ͬ��ͬѹ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�������ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
��12�֣�ij��ѧ��ȤС��Ϊ��̽��п��Ũ���ᷴӦ��������ijɷ���������ʵ�飺
��50gп����50mLŨH2SO4�ڼ��������³�ַ�Ӧ��п����ʣ�࣬�ռ���һ����������壬���������������ɱ�״��Ϊ11.2L��
��1�� ��ѧ��ȤС�����Ƶõ�����X�л��е���Ҫ�������������������������������
�������ʽ�����������ֽ������Ҫԭ���ǣ������ӷ���ʽ��ʾ������������������
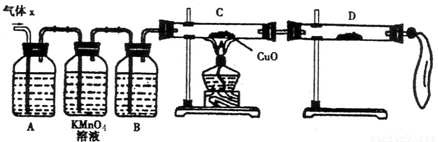
��2��ʵ����֤��Ϊ�˼�����Ҫ��������ijɷ֣���ѧ��ȤС���ͬѧ���������ʵ�飬������Xȡ������̽����
��A�м�����Լ�����������������������������������������������������
��B�м�����Լ�������������������
��֤ʵ����X�л����������壬D��Ӧѡ����Լ���������������������ͬʱӦ�۲쵽C�е�ʵ������������������ ��������������������������
��3�����۷�����
�ٸ�С����ͬѧ���ֻ��Ҫ�ٲ��һ�����ݣ�����ȷ��ȷ�����������ɣ�����Ϊ�������Dz����� ��
A����Ӧ��ʣ��п������Ϊ17.5g
B���ռ������������Ϊ25.8g
C��Ũ��������ʵ���Ũ��Ϊ18.0mol/L
�ڸ������ڢ�����ѡ���ݣ�ͨ������ȷ������X�и��ɷ����ʵ����ֱ�Ϊ����������������������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09-10��Ƹ���ѧ��һ��ѧ����ĩ���Ի�ѧ�� ���ͣ�ʵ����
��8�֣�ij��ѧ��ȤС��Ϊ��̽��п��Ũ���ᷴӦ��������ijɷ���������ʵ�飺
��50gп����50mLŨH2SO4�ڼ��������³�ַ�Ӧ��п����ʣ�࣬�ռ���һ���������
�壬���������������ɱ�״��Ϊ11.2L��
��1����ѧ��ȤС�����Ƶõ�����X�л��е���Ҫ�������������__________���������ֽ������Ҫԭ���ǣ������ӷ���ʽ��ʾ��_________________ ��
��2��ʵ����֤��Ϊ�˼�����Ҫ��������ijɷ֣���ѧ��ȤС���ͬѧ���������ʵ�飬������Xȡ������̽����
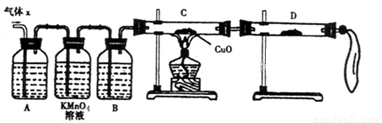
��A�м�����Լ�������__________��B�м�����Լ�������_________��
�ڹ۲쵽C�е�ʵ��������____________________��D�����ѡ����Լ���________��
��3�����۷���������С��ͬѧ�ռ������������Ϊ25.8g������Ũ��������ʵ���Ũ��Ϊ18.0mol/L����ͨ������ȷ������X�и��ɷ����ʵ����ֱ�Ϊ_____________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com