
�ݻ���Ϊ1L�ļס����������������У��ֱ����2molA��2molB��1molA��1molB����ͬ�����£��������з�Ӧ��A(g)��B(g)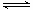 xC(g) ��H��0�������������c(A)��ʱ��t�ı仯����ͼ��ʾ������˵���������
xC(g) ��H��0�������������c(A)��ʱ��t�ı仯����ͼ��ʾ������˵���������
A����������A�ķ�Ӧ����Ϊ0.1mol/L·min
B������������ѹ�����̷�Ӧ�ﵽƽ���ʱ��
C�������������¿����̷�Ӧ�ﵽƽ���ʱ��
D�����ҵ�ƽ��ת�������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧΪ����֤�����к��е�Ԫ��,���������ʵ��,�����������⡣
(1)��1��:���ա������ǽ������������ճɻҽ����ù����в����õ���ʵ����������������(�����)��
A.�Թܡ�B.��������C.����ǯ��D.�����ż�
E.�����ǡ�F.�ƾ��ơ�G.�ձ���H.��Ͳ
(2)��2��:I-��Һ�Ļ�ȡ���������� ��
(3)��3��:���������������μ�����ʵ��Լ����������������ѡ����������(�����)��
A.Ũ���� B.������ˮ
C.KMnO4��Һ D.H2O2
�������� ��
(4)��4��:�ⵥ�ʵļ��顣������ȡ������3������Һ,�μӵ�����Һ,�����Һ����ɫ,��֤�������к��е�Ԫ�ء�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ϩ�;���ϩ����������ȷ���ǡ�(����)
A.��ϩ�Ǵ�����,������Ϊ��̬,����ϩΪ��̬,�ǻ����
B.��ϩ�;���ϩ���ʾ�����,�����ӳɷ�Ӧ
C.ȡ����������ϩ�;���ϩ��ȫȼ�պ�,���ɵ�CO2��H2O�������ֱ����
D.ȡ�����ʵ�������ϩ�;���ϩ,��ȫȼ�պ����ɵ�CO2��H2O�����ʵ����ֱ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һ�ݻ������2 L�ܱ������г���4 mol NH3��
3 mol O2,4 min��,������ɵ�H2Oռ������������40%,�����б�ʾ�˶�ʱ��
�ڸ÷�Ӧ��ƽ�����ʲ���ȷ���ǡ�(����)
A.v(N2)=0.125 mol��L-1��min-1
B.v(H2O)=0.375 mol��L-1��min-1
C.v(O2)=0.225 mol��L-1��min-1
D.v(NH3)=0.250 mol��L-1��min-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ӦA(g)��3B(g)===2C(g)��2D(g)�����ֲ�ͬ����µķ�Ӧ���ʷֱ�Ϊ
��v(A)��0.45 mol·L��1·min��1 ��v(B)��0.6 mol·L��1·s��1
��v(C)��0.4 mol·L��1·s��1 ��v(D)��0.45 mol·L��1·s��1
�����йط�Ӧ���ʵıȽ�����ȷ����
A����>�ۣ���>�� B����<�ۣ���<��
C����>��>��>�� D����>��>��>��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ�˸�������ȡ�����NH3�����з����к��ʵ���(����)
A��N2��3H2 2NH3�����ռ���и���
2NH3�����ռ���и���
B������NH4HCO3��������������������
C������Ũ��ˮ�������ü�ʯ�Ҹ���
D��Mg3N2��6H2O===3Mg(OH)2����2NH3������������ˮ�Ȼ��Ƹ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧΪ��̽�����������ķ�Ӧ�����װ�����ͼ��ʾ��װ�ã����������ѹǿ�� ��ʱ���ˮ��Һ��Ŀ̶�(�ú���)��ȡ���������ڲ���ȼ�ճ��м�����ۣ��þƾ��Ƶ�ȼѸ��������ƿ�в�������������۰�����ȼ�գ�ˮ����������ܡ�������Ϩ����ã�ˮ�����������ػص�ԭ�ȱ궨�Ŀ̶ȡ���Ҫ�ش��������⣺
��ʱ���ˮ��Һ��Ŀ̶�(�ú���)��ȡ���������ڲ���ȼ�ճ��м�����ۣ��þƾ��Ƶ�ȼѸ��������ƿ�в�������������۰�����ȼ�գ�ˮ����������ܡ�������Ϩ����ã�ˮ�����������ػص�ԭ�ȱ궨�Ŀ̶ȡ���Ҫ�ش��������⣺
(1)ˮ������������Ƿ�һ��˵�������������һ�����ڲμӷ�Ӧ���������˵��ԭ��
________________________________________________________________________
____________________��
(2)���δȼ��ʱ�����Ϩ���ˣ�˵��______________________________________
________________________________________________________________________��
(3)����ˮ��������ֻص�ԭ�ȱ궨�Ŀ̶ȣ��ɵó�ʲô���ۣ�
________________________________________________________________________
________________________________________________________________________��
(4)���ݷ�Ӧ����ʽS��O2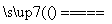 SO2�����������ֿ��Ƶ���ʲô��(��֤��ʲô��)_____________________________________________________________________��
SO2�����������ֿ��Ƶ���ʲô��(��֤��ʲô��)_____________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧ������ͼһ��ʾ��װ����̽��CO2��SO2�����ʯ��ˮ�ķ�Ӧ�����ͨ��CO2���Կ����Ȼ��Ǻ���������ͨ��SO2û�п�������������˼��������ͬѧ������ͼ����װ�ã��������ռ���ע�����������ؽ�����һ������һ�����ݵ�ͨ�����ʯ��ˮ�У����ܿ���ʯ��ˮ�ȱ�����ٱ�����������ͨ��SO2�����������Ա�ͨ��CO2�졣

��1���Աȷ�������ʵ�飬����Ϊ��ͼһװ��ʵ��ʱ��ͨ��SO2���ܳ��ֻ��ǵ�ԭ�������________________________________________________________________________��
��2����ͼ��װ��ʵ��ʱ������ͬ����ͨ��CO2��SO2��SO2�������ǡ�����������CO2���ԭ����_______________________________________________________________��
��3����ͼһ����SO2��ʯ��ˮ��Ӧ��ʵ��ʱ���Ӱ�ȫ�Ƕ� ����װ��Ӧ���θĽ���_____________________________________________________________________��
����װ��Ӧ���θĽ���_____________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com