

| �� |
| �� |
| �� |
| �� |
 ��
�� ��
��| 8.8g |
| 44g/mol |
| �� |
| �� |
| �� |
| �� |


 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ũ���� |
| �� |
| Ũ���� |
| �� |
| һ������ |
| һ������ |
| ������ |
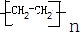
| ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
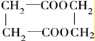
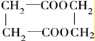
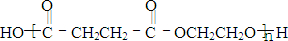
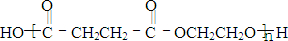
| Cu |
| �� |
| ���� |
| �� |
| Cu |
| �� |
| ���� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
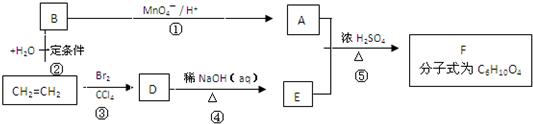
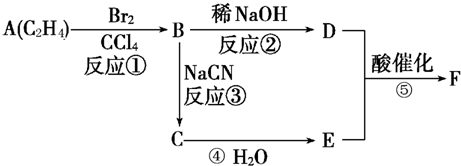
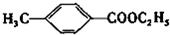 �������ںϳ�ҩ����м��壮���������ת����ϵͼ�ش��й�����
�������ںϳ�ҩ����м��壮���������ת����ϵͼ�ش��й�����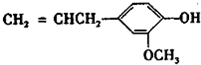 ���ǶԼ�������������ͬ���칹�壬�������������ܷ�����Ӧ����
���ǶԼ�������������ͬ���칹�壬�������������ܷ�����Ӧ����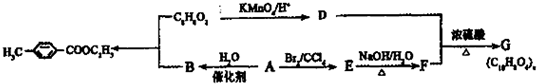
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ��ׯ�и���3��ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ��ƶ���
�Լ������������� �������ںϳ�ҩ����м��塣���������ת����ϵ�ش��й�����
�������ںϳ�ҩ����м��塣���������ת����ϵ�ش��й�����
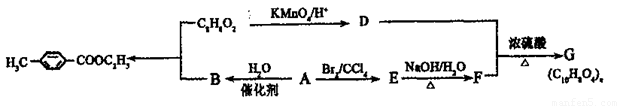
��1��D�к��й����ŵ�������________,A��E�ķ�Ӧ����Ϊ________��
��2��G�Ľṹ��ʽΪ________��
��3��д��1�����������ұ�����ֻ��һ��ȡ������C8H8O2��ͬ���칹��________��
��4������ӣ�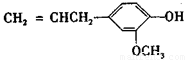 ���ǶԼ�������������ͬ���칹�壬�������������ܷ�����Ӧ����________ (�����)��
���ǶԼ�������������ͬ���칹�壬�������������ܷ�����Ӧ����________ (�����)��
A. NaOH��Һ B.NaHCO3��Һ C.KMnO4/H+ D.FeCl3��Һ
��5��д���ϳɶԼ������������Ļ�ѧ����ʽ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
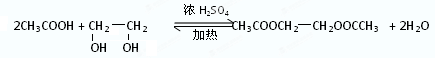
��֪��������軯�Ʒ�Ӧ������ˮ����Եõ����
CH3CH2Br��NaCN��CH3CH2CN��NaBr��CH3CH2CN��H2O��CH3CH2COOH
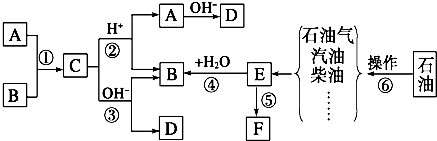
��NH3(δ��ƽ)��������ӱ�ԭ��������Ӷ���һ��̼ԭ�ӣ�������̼�������������ת����ϵ�ش����⡣����F�����к���һ����8��ԭ����ɵĻ�״�ṹ��
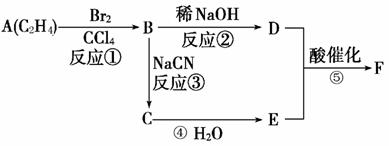
(1) ��Ӧ�١��ڡ���������ȡ����Ӧ����________(�Ӧ����)��
(2) B������Ϊ ��E��F�Ľṹ��ʽΪ��E______________��F________________________��
(3) д��D��E��һ�������·����ۺϷ�Ӧ�IJ���ýṹ��ʽ��
ʾ�� ��
��4��E��ͬϵ����̼ԭ�������ٵ�Ϊ ������ƺ�����������D�ϳɸ����ʣ�Ҫ���û�ѧ����ʽ��ʾ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com