
����Ŀ��̼Ԫ�ز������γɷḻ��ʵ��л���������һ����γɶ�������������C��ͬʱ���������γɶ��ֵ�����D��E��̼���仯�������;�㷺��

��֪AΪ���Ӿ��壬BΪ�������壬CΪ���Ӿ���
(1)ͼ�зֱ���������ֳ����ľ��壬�ֱ��ǣ�A________��B________��C________��D________��E________��(�����ƻ�ѧʽ)
(2)�ɱ��ͱ������ֳ����ķ��Ӿ��壬�������־���ıȽ�����ȷ����_____��
a.������ܶȣ��ɱ�>�� b.������۵㣺�ɱ�>��
c.�����еĿռ������ʣ��ɱ�>�� d.�����з��Ӽ��������������ͬ
(3) ���ʯ��ʯī��̼�����ֳ������ʣ�����������ȷ����________��
a.���ʯ��̼ԭ�ӵ��ӻ�����Ϊsp3�ӻ���ʯī��̼ԭ�ӵ��ӻ�����Ϊsp2�ӻ�
b.�����й��ۼ��ļ��������ʯ��C��C<ʯī��C��C
c.������۵㣺���ʯ>ʯī
d.�����й��ۼ��ļ��ǣ����ʯ>ʯī
e.���ʯ������ֻ���ڹ��ۼ���ʯī����������ڹ��ۼ����������ͷ��»���
f.���ʯ��ʯī���۵㶼�ܸߣ����Խ��ʯ��ʯī����ԭ�Ӿ���
(4)���ʯ�����ṹ��ͼ��һ�������е�Cԭ����ĿΪ ________��
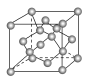
(5)C���ȸʯ���ȿ��Եõ�����ͭ������ͭ���������������ܶѻ�����֪Cu���ʵľ����ܶ�Ϊ�� g/cm3��Cu�����ԭ������ΪM�������ӵ�����ΪNA����Cu��ԭ�Ӱ뾶Ϊ __________cm��
���𰸡�NaCl Na �ɱ� ���ʯ ʯī ac ae 8 ![]() ��
��![]() cm
cm
��������
��1������ͼ�о���Ľṹ����ϳ��������֪��AΪ�Ȼ��ơ�BΪNa��CΪ�ɱ���DΪ���ʯ��EΪʯī��
��2��ˮ���Ӽ���������������з����ԣ�����ˮ�����γɱ�ʱ���ڽϴ�Ŀ�϶���������ڻ�ʱ������ƣ��ɱ�����֮��ֻ���ڷ��»������γɵķ��Ӿ������ܶѻ���
��3��a�����ʯ��̼ԭ�����ĸ�̼ԭ���γ�4�����۵����������������壬ʯī�е�̼ԭ�������ڵ�����̼ԭ���ԦҼ���ϣ��γ�ƽ���������νṹ��
b��c��sp2�ӻ��У�s����ijɷֱ�sp3�ӻ����࣬����ʯī��̼ԭ�ӻ��д�м������γɵĹ��ۼ����̣����ι̣���ʯī�IJ��ڹ��ۼ������Ƚ��ʯ�ļ����̣������������ƻ���ѧ����Ҫ����������
d�����ʯ��̼ԭ�����ĸ�̼ԭ���γ�4�����۵����������������壬ʯī�е�̼ԭ����sp2�ӻ���������ڵ�����̼ԭ���ԦҼ���ϣ��γ��������ε�ƽ���״�ṹ��
e�����ʯ��̼ԭ�����ĸ�̼ԭ���γ�4�����۵����������������壬������ֻ���й��ۼ���ʯī�е�̼ԭ����sp2�ӻ���������ڵ�����̼ԭ���ԦҼ���ϣ��γ��������ε�ƽ���״�ṹ����ÿ��̼ԭ�ӻ���һ��2p�����������һ��2p���ӣ���Щp����ֶ�����ƽ�У�����ֱ��̼ԭ��sp2�ӻ�������ɵ�ƽ�棬�γ��˴�м��������Щ�е��ӿ���������̼ԭ��ƽ���ϻ�����ƽ����������ʣ�ʯīΪ��״�ṹ�������֮��ͨ�����»������ӣ�
f��ʯīΪ��״�ṹ�������֮��ͨ�����»������ӣ�
��4���ɽ��ʯ�ľ����ṹ��֪�������ڲ���4��Cԭ�ӣ���������6��Cԭ�ӣ�������8��Cԭ�ӣ����ݾ�̯�����㣻
��5������ͭ���������������ܶѻ������þ�̯�����㾧��ԭ����Ŀ��ͭԭ�Ӱ뾶Ϊrcm���ɼ��㾧������������m=��V����ͭԭ�Ӱ뾶��
��
��1������ͼ�о���Ľṹ����ϳ��������֪��AΪ�Ȼ��ơ�BΪNa��CΪ�ɱ���DΪ���ʯ��EΪʯī��
��Ϊ��NaCl��Na���ɱ������ʯ��ʯī��
��2��a��ˮ���Ӽ���������������з����ԣ�����ˮ�����γɱ�ʱ���ڽϴ�Ŀ�϶���ܶȱ�ˮС���ɱ�����֮��ֻ���ڷ��»������γɵķ��Ӿ������ܶѻ����ܶȱ�ˮ��a��ȷ��
b�����ڻ�ʱ������ƣ��ɱ�����֮��ֻ���ڷ��»������ڻ�ʱ�ƻ����»���������ȷ��»���ǿ���ʾ�����۵�����ɱ�����b����
c��ˮ���Ӽ���������������з����ԣ�����ˮ�����γɱ�ʱ���ڽϴ�Ŀ�϶���ɱ�����֮��ֻ���ڷ��»������γɵķ��Ӿ������ܶѻ��������еĿռ������ʣ��ɱ���������c��ȷ��
d���ɱ�����֮��ֻ���ڷ��»�����ˮ����֮��ȴ��ڷ��»����ִ�������������з��Ӽ�����������Ͳ���ͬ����d����
��ѡ��ac��
��3��a�����ʯ��̼ԭ�����ĸ�̼ԭ���γ�4�����۵����������������壬̼ԭ�ӵ��ӻ�����Ϊsp3�ӻ���ʯī�е�̼ԭ�������ڵ�����̼ԭ���ԦҼ���ϣ��γ�ƽ���������νṹ��̼ԭ�ӵ��ӻ�����Ϊsp2�ӻ�����a��ȷ��
b��sp2�ӻ��У�s����ijɷֱ�sp3�ӻ����࣬����ʯī��̼ԭ�ӻ��д�м������γɵĹ��ۼ����̣����ι̣���ʯī�IJ��ڹ��ۼ������Ƚ��ʯ�ļ����̣���b����
c��ʯī�IJ��ڹ��ۼ������Ƚ��ʯ�ļ����̣������������ƻ���ѧ����Ҫ�������������Ծ�����۵���ʯ��ʯī����c����
d�����ʯ��̼ԭ�����ĸ�̼ԭ���γ�4�����۵����������������壬����Ϊ109��28�䣬ʯī�е�̼ԭ����sp2�ӻ���������ڵ�����̼ԭ���ԦҼ���ϣ��γ��������ε�ƽ���״�ṹ������Ϊ120�㣬��d����
e�����ʯ��̼ԭ�����ĸ�̼ԭ���γ�4�����۵����������������壬ʯī�е�̼ԭ����sp2�ӻ���������ڵ�����̼ԭ���ԦҼ���ϣ��γ��������ε�ƽ���״�ṹ����ÿ��̼ԭ�ӻ���һ��2p�����������һ��2p���ӣ���Щp����ֶ�����ƽ�У�����ֱ��̼ԭ��sp2�ӻ�������ɵ�ƽ�棬�γ��˴�м��������Щ�е��ӿ���������̼ԭ��ƽ���ϻ�����ƽ����������ʣ�ʯīΪ��״�ṹ�������֮��ͨ�����»������ӣ�˵�������к��й��ۼ��������������»�������e��ȷ��
f�����ʯ��ԭ�Ӿ��壬ʯīΪ��״�ṹ�������֮��ͨ�����»������ӣ�ʯīΪ����;��壬������ԭ�Ӿ��壬��f����
��ѡ��ae��
��4���ɽ��ʯ�ľ����ṹ��֪�������ڲ���4��Cԭ�ӣ���������6��Cԭ�ӣ�������8��Cԭ�ӣ����Խ��ʯ������cԭ����ĿΪ4+6��![]() +8��
+8��![]() =8��
=8��
����8��
��5������ͭ���������������ܶѻ���������Cuԭ����ĿΪ8��![]() +6��
+6��![]() =4��ͭԭ�ӵİ뾶Ϊrcm�������ⳤΪ��
=4��ͭԭ�ӵİ뾶Ϊrcm�������ⳤΪ��![]() 4rcm=
4rcm= ![]() rcm������
rcm������![]()
![]() ��ã�r=
��ã�r=
���� cm
cm



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ�������仯��ȷ���÷�Ӧ���Ȼ�ѧ����ʽ��д��ȷ���ǣ� ��

A. 2A(g)��B(g)=2C(g)����H��0
B. 2A(g)��B(g)=2C(g)����H��0
C. 2A��B=2C����H��0
D. 2C=2A��B����H��0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڷ����廯����A���й���Ϣ���£�
��A��̼����������Ϊ77.77%��H����������Ϊ7.40%������Ϊ����
�ڹ����ⶨʱ����ͼ��ӳ��A����Է�������Ϊ108��
��A������������Ũ�������������������ˮ����ζ�IJ��
��A����������Ʒ�Ӧ�ų����������������������Ʒ�Ӧ���Իش�
��1����A����Է��������Ĺ���Ϊ____(����ĸ)��A�����ϵ�һ�������____�֡�
A.������� B.���� C.�˴Ź�����
��2��Aͨ�������ֲ�ͬ���ķ�����ͬ���칹�壬��д�������ֲ�ͬ����ͬ���칹��Ľṹ��ʽ(��дһ��)��____��____��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��_____��
��4��A��ͭ˿�������¼��ȷ�����Ӧ�Ļ�ѧ����ʽΪ____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��[���ʽṹ������]
[Zn(CN)4]2�C��ˮ��Һ����HCHO�������·�Ӧ��
4HCHO+[Zn(CN)4]2�C+4H++4H2O===[Zn(H2O)4]2++4HOCH2CN
��1��Zn2+��̬��������Ų�ʽΪ____________________��
��2��1 mol HCHO�����к�����������ĿΪ____________mol��
��3��HOCH2CN������̼ԭ�ӹ�����ӻ�������______________��
��4����H2O���ӻ�Ϊ�ȵ������������Ϊ________________��
��5��[Zn(CN)4]2�C��Zn2+��CN�C��Cԭ���γ���λ���������ǿռ乹�ͣ�[Zn(CN)4]2�C�Ľṹ����ʾ��ͼ��ʾΪ_____________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪����A��ʯ���ѽ�������Ҫ�ɷݣ�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����2CH3CHO+O2 2CH3COOH������AΪ��Ҫԭ�Ϻϳ�������������ϳ�·������ͼ��ʾ��
2CH3COOH������AΪ��Ҫԭ�Ϻϳ�������������ϳ�·������ͼ��ʾ��

�ش��������⣺
��1��д��A�ĵ���ʽ_______________________��
��2��B��D�����еĹ��������Ʒֱ���_______��________��
��3��д�����з�Ӧ�ķ�Ӧ���ͣ���______����______����_____��
��4��д�����з�Ӧ�Ļ�ѧ����ʽ��
��______________________________________��
��________________________________________��
��_________________________________________��
��5��������뺬���ᡢ�Ҵ���ˮ�����������ֲ�Ʒ����ͼ�Ƿ��������������ͼ������ͼ��Բ�����������ʵ����Լ����ڷ������������ʵ��ķ��뷽����

�����Լ�a��__________���Լ�b��_______________��
���뷽������_________�����뷽������____________�����뷽������___________��
�����ڵõ���A�м�����ˮ̼���Ʒ�ĩ����Ŀ����______________��
����д��C �� D ��Ӧ�Ļ�ѧ����ʽ________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ϩ���������в�����ζ��������ʳƷ���Ӽ�����ҵ�Ͽ�������ϩ����ϩ��Ϊԭ�Ϻϳ��Ƶá�
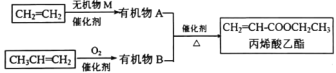
(1)��CH2=CH2�����л���A(����ʽΪC2H6O)�ķ�Ӧ������____��
(2)��ϩ������(CH2=CH-COOCH2CH3)�к��������ŵ�����_______��
(3)�л���B�Ľṹ��ʽΪ______��������ϩ������Ľṹ�����ʽ�����ȣ������л���B��˵����ȷ����____��
a �л���B��CH3COOH���еĹ�������ȫ��ͬ
b ������NaHCO3��Һ��Ӧ�ų�CO2����
c ��һ�������¿��Է����������ӳɡ�������Ӧ
(4)�л���A��B��Ӧ���ɱ�ϩ�������ķ�Ӧ��ʵ�����п�����ͼװ���н��С�

�ٸ÷�Ӧ�Ļ�ѧ����ʽ��___________
���Թ������Լ���������_________����Ҫ���ƵõIJ��������������õ�ʵ�������______(������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧ��Ϊ̽��п�����ᷴӦ�����е����ʱ仯����![]() ʱ����100 mL 2 mol��L-1�����м��������п�ۣ�����������(�ѻ���ɱ�״��)�ۼ�ֵ���£�
ʱ����100 mL 2 mol��L-1�����м��������п�ۣ�����������(�ѻ���ɱ�״��)�ۼ�ֵ���£�
ʱ��(min) | 1 | 2 | 3 | 4 | 5 |
�������(mL) | 50 | 120 | 232 | 290 | 310 |
(1)����2~3 minʱ����ڣ��������Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ_______.
����0~5min�ڣ���Ӧ��������ʱ�����___(�� ��1~2 min������2~3 min������3~4 min��)��
(2)����ȫ��Ӧ��ų�15.2 kJ����������ӦZn(s) + 2HCl(aq)=ZnCl2(aq) + H2(g)����H=____
(3)Ϊ�˼�����Ӧ���ʵ������ٲ����������������Ӧ���зֱ��������������Һ�壬����Ϊ���е���_______(����ĸ)��
a. ����ˮ b. Na2CO3��Һ c. NaNO3��Һ
(4)Ϊ�˼ӿ췴Ӧ���ʵ������ٲ������������ijͬѧ��Ӧ���м���������CuSO4���壬��ͬѧ����____(�� ��������������������)��������_______
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CH4����һ����Ҫ����Դ��Ҳ��һ����Ҫ�Ļ���ԭ�ϡ�
(1)������·ֽ�����������̼�����ܱ������н��д˷�Ӧʱ��Ҫͨ����������ʹ���ּ���ȼ�գ���Ŀ����____________����֪25�桢101 kPaʱ��1 mol������ȫȼ������CO2��Һ̬ˮʱ�ų�896 kJ��������������ֵΪ___kJ��g-1��
(2)һ���¶��£���ƫ������ͭ(CuAlO2)�Ĵ������£�CH4��CO2ת�������ᣬ��ʵ����CO2��������д����Ӧ�Ļ�ѧ����ʽ__________�����ŵ���_____(��д��һ��)��
(3)���������������������Ⱦ����: CH4(g)+2NO2(g)![]() N2(g) + CO2(g) + 2H2O(g)��
N2(g) + CO2(g) + 2H2O(g)��
�����д�ʩ�ܹ��ӿ컯ѧ��Ӧ���ʵ���______��
a. ʹ�ô��� b. �����¶� c. ��ʱ����ˮ
����������Ӧ�ں����ܱ������н��У�������������˵���÷�Ӧ��ƽ�����_____��
a. ���������������ٱ仯
b. c(NO2) = 2c(N2)
c. ��λʱ��������1 mol CO2��ͬʱ����2 mol NO2
(4)�����ֱ��Ӧ����ȼ�ϵ�أ��õ�ز��ÿɴ���O2-�Ĺ���������Ϊ����ʣ��乤��ԭ����ͼ��ʾ:

��b���缫��ӦʽΪ_________��
����ȼ�ϵ�����ĵĿ����ڱ�״���µ������5.6L(���������O2�������Ϊ20%)�������������ļ���____mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ݱ�Ţ�~�������Ԫ�������ڱ��е�λ�ã��ش��������⣺
�� | �� | �� | �� | �� | �� | �� | 0 | |
1 | �� | �� | ||||||
2 | �� | �� | �� | �� | ||||
3 | �� | �� | �� | �� |
(1)����+1�ۣ�������-1�۵�Ԫ����____(��Ԫ�ط���):�ڵķ���ʽ��____
(2)����߰�ԭ�Ӹ�����1:1�γɻ�����ף������ʽΪ____������еμ�����ˮʱ������Ӧ�Ļ�ѧ����ʽ��____
(3)�ߡ��ࡢ������Ԫ������������Ӧ��ˮ���������ǿ������˳������Ϊ____(�ѧʽ);�������������Ӧ��ˮ����Ļ�ѧʽΪ____
(4)�١��ݡ�������Ԫ���γɵ�һ�ֳ����εĻ�ѧʽΪ____/span>�����к��еĻ�ѧ��Ϊ____
(5)���������ɢ�����γɵĻ�����ʱ�������____ɫ��������ں��պ���Ͻ���ϵ��Ʊ�����ҵ��ұ���õ��ʵĻ�ѧ����ʽΪ____
(6)Ԫ�آ�ĵ��ʺ͢ߵ�����������ˮ����֮�䷢����Ӧ�����ӷ���ʽΪ____
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com