
 2KNO2+O2����
2KNO2+O2���� 2CuO+2NO2��+O2����4AgNO3
2CuO+2NO2��+O2����4AgNO3 4Ag+4NO2��+O2��
4Ag+4NO2��+O2�� M��NO2��2+O2��
M��NO2��2+O2�� 2MO+4NO2��+O2��
2MO+4NO2��+O2��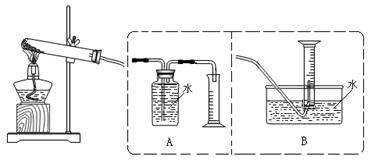
 M+2NO2��+O2����3�֣���2��B ��2�֣�װ��A��Ӧ�����ܽ���������������ˮ��������ˮ�ܿ�Ӧ������Ͳ���²����Ա����ǰ��ƽҺ�棨2�֣�
M+2NO2��+O2����3�֣���2��B ��2�֣�װ��A��Ӧ�����ܽ���������������ˮ��������ˮ�ܿ�Ӧ������Ͳ���²����Ա����ǰ��ƽҺ�棨2�֣�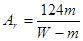 ��3�֣�
��3�֣� M+2NO2��+O2����
M+2NO2��+O2����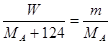 �����MA��
�����MA��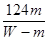 ��
��

 ��������������������ϵ�д�
��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢ� | B���٢ڢܢ� | C���٢ڢܢݢ� | D���٢ڢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
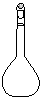 ����������
����������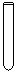 ����������
����������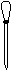 ����������
����������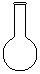 ����������
����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢݢ� | B���٢ۢݢ� | C���ڢۢܢ� | D���ۢܢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
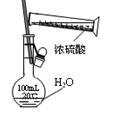 | 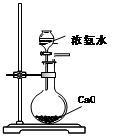 | 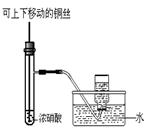 |  |
| A������һ��Ũ������ | B���Ʊ��������� | C���Ʊ����ռ�����NO2���� | D���Ʊ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����õ�NaOH �ѳ��� |
| B���ô������������ƽ����NaOH��������ʱ��������������̣���������������� |
| C��������ƽ���������ϸ���һ��ֽ��������ƽ��ƽ���NaOH�������ֽ�ϳ��� |
| D������Ͳ��ȡˮʱ������ˮ��̶�������ȡ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A����˫��ˮ�Ͷ���������ȡ���� |
| B��ʳ����Һ������ȡ�Ȼ��ƾ��� |
| C���Ȼ�����ʯ�ҷ�Ӧ��ȡ���� |
| D��Ũ�����ͭ��Ӧ��ȡ������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ֻ�Т� | B��ֻ�Т�? | C���ڢۢ� | D���٢ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A���ڴ������ڵ������£�������ˮ������Ӧ��������ɫ����ˮ�ص�Һ���屽�� |
| B����ͭ˿�������״���ھƾ����ϼ��ȱ�ں���������ʢ����ˮ�Ҵ����Թ��У�����Ҵ�����Ϊ��ȩ��ʵ�顣 |
| C�����к�������ˮ�ɼ�����ʯ���������Ƶ���ˮ�Ҵ��� |
| D����֤���ǵ�ˮ�����ʱ�������Ǻ�ϡH2SO4��Һ��ϣ�ˮԡ����ʹ����ˮ�⣬Ȼ����ϡ��NaOH��Һʹ��ʼ��Ժ��ٵμ�������Һ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com