
�������ֶ�����Ԫ�ص�ijЩ���������ʾ(����ֻ��W��Y��ZΪͬ����Ԫ��)��
| Ԫ�ش��� | X | W | Y | Z | Q |
| ԭ�Ӱ뾶(��10��12 m) | 37 | 64 | 66 | 70 | 154 |
| ��Ҫ���ϼ� | ��1 | ��1 | ��2 | ��5����3 | ��1 |
����˵����ȷ����(����)
A����Q��Y�γɵĻ�������ֻ�������Ӽ�
B��Z��X֮���γɵĻ�������л�ԭ��
C����X��Y��Z����Ԫ���γɵĻ�����侧��һ���Ƿ��Ӿ���
D��Y��W�γɵĻ����Y�Ը���
 �߽�������ϵ�д�
�߽�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10.6 g Na2CO3����ˮ���1 L��Һ
(1)����Һ��Na2CO3�����ʵ���Ũ��Ϊ__________����Һ��Na�������ʵ���Ũ��Ϊ__________��
(2)�����Һ�м���һ����NaCl���壬ʹ��Һ��Na�������ʵ���Ũ��Ϊ0.4 mol·L��1(������Һ�������)�����NaCl������Ϊ__________��Cl�������ʵ���Ũ��Ϊ________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ���¶��£��ڹ̶�������ܱ������з������з�Ӧ��2HI��g��=H2��g��+I2��g����C(HI�� ��0.1mol/L����0.07mol/Lʱ����Ҫ15S����ôC(HI)��0.07mol/L����0.05mol/Lʱ�����跴Ӧʱ��Ϊ�� ��
A.����5S B.����10S C ����10S D��10S
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ԫ�����ʵ������Ա仯������(����)
A��ԭ�Ӱ뾶�������������� B�����ϼ�
C��ԭ�Ӻ�����ӽṹ D�������Ժͷǽ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��Z��R��W��5�ֶ�����Ԫ�أ�ԭ�����������������ǿ�������ӻ�����Z2Y���ۻ�����RY3��XW4����֪Y��Rͬ���壬Z��R��Wͬ���ڡ�����˵������ȷ����(����)
A��ԭ�Ӱ뾶��Z>R>W
B��X2W6�����и�ԭ�Ӿ�����8���ӽṹ
C����̬�⻯����ȶ��ԣ�HmW>HnR
D��Y��Z�γɵĻ�������ֻ���ܴ������Ӽ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��Z��Q��MΪ�����Ķ�����Ԫ�أ���ԭ���������������й���Ϣ���±���
| X | ��ֲ����������ȱ�ٵ�Ԫ�أ��ǵ����ʵ���Ҫ�ɷ� |
| Y | �ؿ��к����ӵ�һλ |
| Z | ����������ԭ�Ӱ뾶��� |
| Q | �����д���ʹ����Ͻ���Ʒ����ҵ�Ͽ��õ����������ķ����Ʊ� |
| M | ��ˮ�д���������Ԫ��֮һ������������븺�۵Ĵ�����Ϊ6 |
(1)X����̬�⻯��Ĵ���������������˵���������ʳ��������µļ������������⣬��д������̬�⻯��ĵ���ʽ��____��
(2)��֪37Rb��53I��λ�ڵ������ڣ��ֱ���Z��Mλ��ͬһ���塢�����й�˵����ȷ����________________(�����)��
A��ԭ�Ӱ뾶��Rb>I
B��RbM�к��й��ۼ�
C����̬�⻯�����ȶ��ԣ�M >I
D��Rb��Q��M������������Ӧ��ˮ�����������������Ӧ
(3)X��Y��ɵ�һ����ɫ������������Ϊ����ɫ������״����40 L����ɫ������15 L����ͨ��һ��Ũ�ȵ�NaOH��Һ�У�ǡ�ñ���ȫ���գ�ͬʱ���������Ρ���д���÷�Ӧ�����ӷ���ʽ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������У��Ⱥ������Ӽ����ֺ��м��Լ����� �� ��
A��CO2 B��KOH C�� CaCl2 D��H2O2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��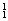 H��
H��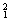 H��
H��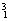 H��
H�� 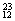 Mg��
Mg��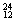 Mg��
Mg�� O��
O��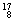 O��
O��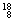 O�й���______��Ԫ�أ�______�ֺ��أ������������� ��D218O����Է���������______��
O�й���______��Ԫ�أ�______�ֺ��أ������������� ��D218O����Է���������______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������������������1:1�����һ�Թ��в�������ˮ������ڹ���������ͼ��ʾ�����������У�����ȷ����
A.ƿ������Ļ���ɫ��dz B.ƿ�ڱ�����״Һ���γ�
C.�˷�Ӧ��������ֻ��һ�ȼ��� D.�Թ���Һ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com