
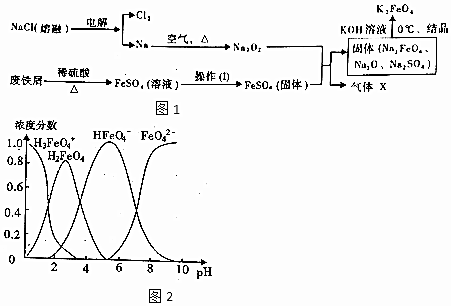
���� ��1��ʪ���Ʊ�������أ�K2FeO4������FeO42-Ϊ���Fe��OH��3Ϊ��Ӧ����ϼ����ߣ���Ԫ�ػ��ϼ۽��ͣ���ClO-����Cl-���ݴ˷����ɵã�
��2����������Ȼ��Ƶõ������ͽ����ƣ����ڿ�����ȼ�����ɹ������ƣ�����м����ϡ������ȷ�Ӧ�õ�����������Һ��ͨ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӵõ������������壬����������ѹ����õ������������壬������������������ƻ�Ϸ�Ӧ�õ�����Na2FeO4��Na2SO4��Na2O�������ܽ�Ȳ����������������Һ�ᾧ�õ�K2FeO4��ͬʱ������XΪO2��
�ٲ�����������������Һ�еõ�������������Ĺ�����Ҫ����Ũ������ȴ�ᾧ����ϴ�ӣ�����������ѹ����õ������������壻
�ڹ��̷�����֪XΪ������
��3����K2FeO4��Һ����ǿ�����ԣ�Ӧ����ʽ�ζ�������ȡ��
����ǿ������Һ�У��ù���CrO2-��FeO42-��Ӧ����Fe��OH��3��CrO42-����ϵ���غ��ԭ���غ���ƽ��д���ӷ���ʽ��
��K2Cr2O7��ҺΪ��ɫ��Fe3+����ҺΪ��ɫ��
��CrO2-+FeO42-+2H2O=Fe��OH��3��+CrO42-+OH-��6Fe2++Cr2O72-+14H+�T6Fe3++2Cr3++7H2O�����ݷ�Ӧ�Ķ�����ϵ��Ԫ���غ���㣻
��4��A��PH=2ʱ��������غ�����жϣ�
B������ͼƬ֪���ı���Һ��pH������Һ��pH=10����pH=4�Ĺ����У�HFeO4-�ķֲ�������������С��
C����pH=1����Һ�м�HI��Һ��HI���л�ԭ�Ա�������������
D����ͬPHֵʱ����Һ����Ԫ�صĴ�����̬����������ͬ��������Һ�д���FeO42-��
��� �⣺��1��ʪ���Ʊ�������أ�K2FeO4������Fe��OH��3����FeO42-Ϊ�����Ԫ�ػ��ϼ����ߣ��ɵ���ת���غ��֪����Ԫ�ػ��ϼ۽��ͣ���ClO-����Cl-�����ӷ���ʽΪ��2Fe��OH��3+3ClO-+4OH-=2FeO42-+3Cl-+5H2O���÷�Ӧ�������뻹ԭ�����ʵ���֮��Ϊ3��2��
�ʴ�Ϊ��3��2��
��2��������I������Һ�еõ�������������ķ���Ϊ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӣ�
�ʴ�Ϊ������Ũ������ȴ�ᾧ��
�ڹ��̷�����֪XΪ���������������ķ���Ϊ���ô����ǵ�ľ���Ӵ����壬�����ľ����ȼ֤����������
�ʴ�Ϊ���ô����ǵ�ľ���Ӵ����壻
��3����K2FeO4��Һ����ǿ�����ԣ�ȷ��ȡ25.00mL K2FeO4��Һ���뵽��ƿ��Ӧ����ʽ�ζ�������ȡ��
�ʴ�Ϊ����ʽ�ζ��ܣ�
����ǿ������Һ�У��ù���CrO2-��FeO42-��Ӧ����Fe��OH��3��CrO42-�����ӷ���ʽΪ��CrO2-+FeO42-+2H2O=Fe��OH��3��+CrO42-+OH-��
�ʴ�Ϊ��CrO2-+FeO42-+2H2O=Fe��OH��3��+CrO42-+OH-��
�ۼ����������������ָʾ������0.1000mol•L-1 ��NH4��2Fe��SO4�� 2����Һ�ζ����յ���Һ���Ϻ�ɫ������ָʾ����ΪK2Cr2O7��ҺΪ��ɫ��Fe3+����ҺΪ��ɫ����ɫ�仯�����ԣ�
�ʴ�Ϊ������ΪK2Cr2O7��ҺΪ��ɫ��Fe3+����ҺΪ��ɫ����ɫ�仯�����ԣ�
��CrO2-+FeO42-+2H2O=Fe��OH��3��+CrO42-+OH-��6Fe2++Cr2O72-+14H+�T6Fe3++2Cr3++7H2O���õ�������ϵΪ��
2FeO42-��2CrO42-��Cr2O72-��6Fe2+��
2 6
n 0.0300L��0.1000mol/L
n=0.001mol��
100ml��Һ�к���0.001mol��$\frac{100mL}{25mL}$=0.004mol��
�ⶨ����Ʒ��K2FeO4����������=$\frac{0.004mol��198g/mol}{1.0g}$��100%=79.2%��
�ʴ�Ϊ��79.2%��
��4��A��ͼ�������֪pH=2ʱ����Һ�д��������غ㣬������ʽΪ��H3FeO4+��H2FeO4��HFeO4-������0.1mol•L-1��K2FeO4��Һ�д��������غ�Ϊ��c��H3FeO4+��+c��H2FeO4��+c��HFeO4-��=0.1mol•L-1����A��ȷ��
B����pH=10��������Һ�м��������Һˮ�������ԣ�ͼ��仯��֪������ͼƬ֪���ı���Һ��pH������Һ��pH=10����pH=4�Ĺ����У�HFeO4-�ķֲ�������������С����B����
C����pH=1����Һ�м�HI��Һ��HI���л�ԭ�ԣ�������Ӧ���������ӡ������ӱ�����Ϊ�ⵥ�ʣ���Ӧ�����ӷ���ʽ����C����
D����K2FeO4��������ˮ����ͬPHֵʱ����Һ����Ԫ�صĴ�����̬����������ͬ��������Һ�д���FeO42-��FeO42-��������������ˮ��Һ�г������ԣ���D��ȷ��
�ʴ�Ϊ��A��D��
���� ���⿼��ѧ���Ķ���Ŀ��ȡ��Ϣ���������Թ������̵������������Ŀ��ơ������ʵ���Ũ������ȣ��Ѷ��еȣ���Ҫѧ��������ʵ�Ļ���֪ʶ���������֪ʶ��������������ע�����֪ʶ�����գ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ȶ��ԣ�NH3��H2O��H2S | B�� | �����ԣ�Cl2��S��P | ||
| C�� | ���ԣ�H3PO4��H2SO4��HClO4 | D�� | ���ԣ�Mg��OH��2��Ca��OH��2��Ba��OH��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
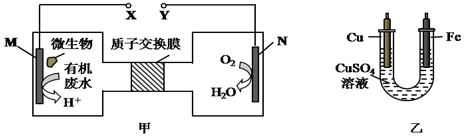
| A�� | ͭ�缫Ӧ��Y������ | |
| B�� | ��װ������Һ����ɫ�����dz | |
| C�� | ��N�缫����0.25 mol����ʱ�������缫��������16 g | |
| D�� | M�缫��Ӧʽ��H2NCONH2+H2O-6e-�TCO2��+N2��+6H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ѧ���õ�����ƽ��ȡNaOH����1.220g | |
| B�� | ��ѧ���ù㷺pH��ֽ�ⶨ��Һ������ԣ�pH=14.5 | |
| C�� | ��ѧ���ü�ʽ�ζ���ȡ25.0mL0��lmol/L������ | |
| D�� | ��ѧ����50mL ��Ͳ��ȡ46.70mLŨ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��̼������Һ�м���������CO32-+2H+�TH2O+CO2�� | |
| B�� | ��ƫ��������Ҵ��ͨ�����������̼��CO2+2H2O+AlO2-�TAl��OH��3��+HCO3- | |
| C�� | ��Ī����[��NH4��2Fe��SO4��2•6H2O]��Һ�м����������������Һ��NH4++Fe2++3OH-�TNH3•H2O+Fe��OH��2�� | |
| D�� | ��˫��ˮ�м������Ը��������Һ��5H2O2+2MnO4-�T2Mn2++5O2��+6OH-+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ú��������Һ����ʯ�͵ķ����Ϊ�����仯 | |
| B�� | ������ˮ�����ɰ�����ķ�Ӧ����ȡ����Ӧ | |
| C�� | ������������Ӧ�ɵõ��е㲻ͬ��4 �ֶ��ȴ��� | |
| D�� | �����Ӳ֬�ᣨC17H35COOH����Ϊͬϵ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
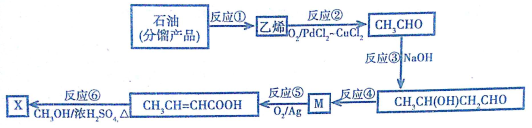
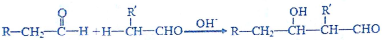
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
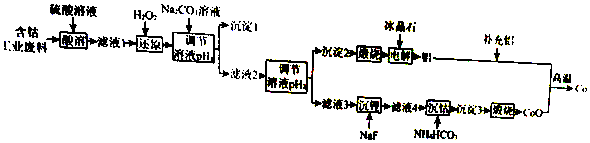
| ���ܷ��ϵijɷ� | |||||
| �ɷ� | Al | Li | Co2O3 | Fe2O3 | ����������ǿ������� |
| ��������/% | 10.5 | 0.35 | 65.6 | 9.6 | 13.95 |
| ��ʵ���в������ӿ�ʼ�����ͳ�����ȫ��pH | |||
| �������� | Fe3+ | Co2+ | Al3+ |
| ��ʼ������pH | 1.9 | 7.15 | 3.4 |
| ������ȫ��pH | 3.2 | 9.15 | 4.7 |
 ]-��
]-���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
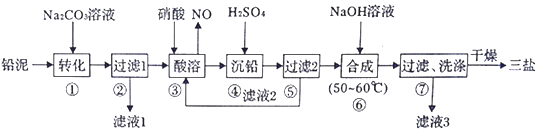
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com