
 N2(g)��2CO2(g)����H�� ��
N2(g)��2CO2(g)����H�� ��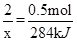 x=1136kJ�����ԣ����Ȼ�ѧ����ʽΪ2N2H4(g)��2NO2(g)��3N2(g)��2H2O (g) ��H����1136kJ/mol
x=1136kJ�����ԣ����Ȼ�ѧ����ʽΪ2N2H4(g)��2NO2(g)��3N2(g)��2H2O (g) ��H����1136kJ/mol

 ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 2CO(g)��
2CO(g)�� 2CO2(g)
2CO2(g)| A��12gC�����е�����һ������28gCO�����е����� |
| B��56gCO��32gO2�����е�������һ������88gCO2�����е������� |
| C��12gC��32gO2�����е�������һ������44gCO2�����е������� |
| D������ͬ������̼ȼ�գ�����CO2������CO�ų��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��450kJ | B��650kJ | C��800kJ | D��520kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 O2(g)===CO(g)����H2
O2(g)===CO(g)����H2 O2(g)===H2O(l)����H5
O2(g)===H2O(l)����H5| A���١��� | B���ܡ��� | C���ڢۢܡ��� | D���٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������¶ȣ������b·�����Ҵ���ת���� |
| B�����������ĵĽǶ�������b·������������� |
| C���Ҵ���ͨ�����۵�����ԭ�Ϸ����Ƶã����ڿ�������Դ |
| D����a��b֪��2H2(g)+O2(g)��2H2O(g)��H=��483.6kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A����֪��H2(g)��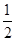 O2(g)===H2O(l)����H����285.8 kJ��mol��1 ����H2��ȼ����Ϊ��285.8 kJ��mol��1 O2(g)===H2O(l)����H����285.8 kJ��mol��1 ����H2��ȼ����Ϊ��285.8 kJ��mol��1 |
| B����֪��S(g)��O2(g)===SO2(g) ��H1����Q1 ��S(s)��O2(g)===SO2(g) ��H2����Q2����Q1<Q2 |
C����֪��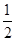 H2SO4(Ũ)��NaOH(aq)="==" H2SO4(Ũ)��NaOH(aq)="==" 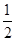 Na2SO4(aq)��H2O(l) ��H1�� Na2SO4(aq)��H2O(l) ��H1��CH3COOH(aq)��NH3��H2O(aq)===CH3COONH4(aq)��H2O(l)����H2������|��H1|<|��H2| |
| D����֪��Fe2O3(s)��3C(ʯī)===2Fe(s)��3CO(g)��H����489.0 kJ��mol��1 |
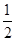 O2(g)===CO2(g)����H����283.0 kJ��mol��1
O2(g)===CO2(g)����H����283.0 kJ��mol��1�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��C���̣�+O2������=CO2�������� ��H ="+393.5" kJ/mol |
B��C���̣�+ O2������=CO�������� ��H ="-393.5" kJ/mol O2������=CO�������� ��H ="-393.5" kJ/mol |
| C��C + O2= CO2����H ="-393.5" kJ/mol |
| D��C���̣�+O2������=CO2�������� ��H ="-393.5" kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
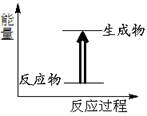
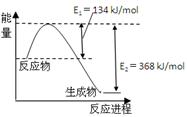
| A����ͼ��ʾ�ķ�ӦΪ���ȷ�Ӧ |
| B����ѧ��Ӧ�������ʱ仯Ҳ�������仯 |
| C����Ҫ���ȵĻ�ѧ��Ӧ��һ�������ȷ�Ӧ |
| D����ѧ������������������ѧ�����ɷų����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������1molH2SO4��Ũ�����뺬��1molNaOH��ϡ��Һ��� |
| B��0.1 mol/L �Ĵ�����Һ�� 1mol/L��NaOH��Һ�������� |
| C��1mol/L������10mL��1mol/L��NaOH15mL��� |
| D��1mol/L������10mL��1mol/L�İ�ˮ15mL��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com