
£Ø16·Ö£©£Ø1£©ĒėĢīŠ“ĻĀĮŠæհד¦£ŗŹµŃéŹŅĄļŅŖÅäÖĘ500mL0.2mol/LµÄĮņĖįÄĘČÜŅŗ”£ŹµŃé²½Öč“óÖĀÓŠ£ŗ
A”¢ŌŚĢģĘ½ÉĻ³Ę³ö___________gĮņĖįÄĘ¹ĢĢ壬°ŃĖü·ÅŌŚÉÕ±Ąļ£¬ÓĆŹŹĮæµÄÕōĮóĖ®Čܽā”£
B”¢°ŃµĆµ½µÄČÜŅŗŠ”ŠÄµŲŃŲ×Å__________×¢Čė________mLµÄČŻĮæĘæÖŠ”£
C”¢ÓĆÉŁĮæÕōĮóĖ®Ļ“µÓÉÕ±ŗĶ²£Į§°ō2”«3“Ī£¬Ćæ“ĪĻ“µÓŅŗŅ²Š”ŠÄ×ŖČėČŻĮæĘæÖŠ”£
D”¢¼ĢŠųĻņČŻĮæĘæÖŠ¼ÓÕōĮóĖ®ÖĮŅŗĆę¾ąæĢ¶Č “¦£¬øÄÓĆ_______________Š”ŠÄµĪ¼ÓÕōĮóĖ®ÖĮČÜŅŗ°¼ŅŗĆęµ×²æÓėæĢ¶ČĻßĖ®Ę½ĻąĒŠ”£
E”¢½«ĘæČūČū½ō£¬³ä·ÖŅ”ŌČ”£
F”¢½«ÅäŗƵÄČÜŅŗµ¹ČėŹŌ¼ĮĘæÖŠ£¬ĢłÉĻ±źĒ©£¬²¢Ļ“µÓČŻĮæĘ攣
£Ø2£©Óė16gŃõĘųĖłŗ¬µÄ·Ö×ÓŹżĻąĶ¬µÄ°±ĘųŹĒ g£¬Óė16gŃõĘųĖłŗ¬Ō×Ó×ÜŹżĻąĶ¬µÄ°±ĘųŹĒ
g£¬ŌŚĻąĶ¬×“æöÖŠ£¬5.6gµŖĘųÖŠÓ¦Ģķ¼Ó g°±Ęų£¬Ėł×é³ÉµÄ»ģŗĻĘųĢåµÄĢå»żÓė16 gŃõĘųĖłÕ¼ÓŠµÄĢå»żĻąµČ”£
£Ø16·Ö£¬ĆææÕ2·Ö£©£Ø1£©A.14.2 B.²£Į§°ō 500 D. l”«2cm ½ŗĶ·µĪ¹Ü
£Ø2£© 8.5g”¢ 4.25g”¢ 5.1g
”¾½āĪö”ææ¼²éŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗµÄÅäÖĘ”£
£Ø1£©500mL0.2mol/LµÄĮņĖįÄĘČÜŅŗÖŠČÜÖŹµÄĪļÖŹµÄĮæŹĒ0.5L”Į0.2mol/L£½0.1mol£¬ÖŹĮæŹĒ0.1mol”Į142g/mol£½14.2g”£×ŖŅʱŲŠė½čÖśÓŚ²£Į§°ō£¬¶ØČŻŹ±ŠčŅŖ½ŗĶ·µĪ¹Ü”£
£Ø2£©16ŃõĘųµÄĪļÖŹµÄĮæŹĒ16g”Ā32g/mol£½0.5mol£¬°±ĘųµÄĪļÖŹµÄĮæŅ²ŹĒ0.5mol£¬ĘäÖŹĮæŹĒ0.5mol”Į17g/mol£½8.5g”£0.5molŃõĘųŗ¬ÓŠ1.0molŌ×Ó£¬ĖłŅŌÓė16gŃõĘųĖłŗ¬Ō×Ó×ÜŹżĻąĶ¬µÄ°±ĘųŹĒ1mol”Ā4£½0.25mol£¬ĘäÖŹĮæŹĒ0.25mol”Į17g/mol£½4.25g”£øł¾Ż°¢·ü¼ÓµĀĀŽ¶ØĀÉæÉÖŖ£¬»ģŗĻĘųµÄĪļÖŹµÄĮæŹĒ0.5mol£¬ĘäÖŠµŖĘųŹĒ5.6g”Ā28g/mol£½0.2mol£¬ĖłŅŌ°±ĘųµÄĪļÖŹµÄĮæŹĒ0.5mol£0.2mol£½0.3mol£¬ĘäÖŹĮæŹĒ0.3mol”Į17g/mol£½5.1g.


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| Į£×Ó“śŗÅ | A | B | C | D | E | F | G |
| Ō×ÓŗĖøöŹż | µ„ŗĖ | µ„ŗĖ | Ė«ŗĖ | ĖÄŗĖ | µ„ŗĖ | ĪåŗĖ | ĪåŗĖ |
| µēŗÉŹż | 0 | 1+ | 1- | 0 | 2+ | 1+ | 0 |
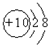
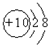


| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ĪļÖŹ | FeSO4 | H2SO4 | Ag2SO4 | Al2£ØSO4£©2 | ĪŪÄą |
| ÖŹĮæ·ÖŹż/£Ø%£© | 15.0 | 7.0 | 0.40 | 0.34 | 5.0 |
| ĪĀ¶Č/”ę | 0 | 10 | 20 | 30 | 40 | 50 |
| FeSO4ČÜŅŗ¶Č£Øg£© | 15.6 | 20.5 | 26.5 | 32.9 | 40.2 | 48.6 |
| Al2£ØSO4£©3Čܽā¶Č£Øg£© | 31.2 | 33.5 | 36.4 | 40.4 | 45.7 | 52.2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻņŅ»øöĢå»żæɱäµÄĆܱÕČŻĘ÷ÖŠ³äČė4mol A”¢1mol B£¬·¢ÉśČēĻĀ·“Ó¦£ŗ4A(g)£«B(s)??3C(s)£«4D(g)”£ŌŚøßĪĀĻĀ“ļµ½Ę½ŗā£¬²āµĆ»ģŗĻĘųĢåÖŠDµÄÅضČĪŖ0.3mol”¤L£1”£ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
(1)ČōČŻĘ÷Ģå»żĪŖ10L£¬·“Ó¦¾2min“ļĘ½ŗā£¬ŌņŅŌAĪļÖŹÅØ¶Č±ä»Æ±ķŹ¾µÄ»Æѧ·“Ó¦ĖŁĀŹĪŖ””””””””””””””””””£¬“ļĘ½ŗāŹ±AĪļÖŹµÄ×Ŗ»ÆĀŹĪŖ”””””””””””””£
(2)ČōŃ¹ĖõČŻĘ÷Ōö“óŃ¹Ē棬ŌņÄę·“Ó¦µÄĖŁĀŹ””””””””£¬ČŻĘ÷ÖŠDµÄĢå»ż·ÖŹż””””””””(Ģī”°Ōö“ó”±”°¼õŠ””±»ņ”°²»±ä”±)”£
(3)ČōĻą¶Ō·Ö×ÓÖŹĮæM(B)>3M(C)£¬ĪĀ¶ČÉżøߏ±»ģŗĻĘųĢåµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæ¼õŠ”£¬ŌņÕż·“Ó¦””””””””(Ģī”°ĪüČČ”±»ņ”°·ÅČČ”±)”£
(4)ŌŚ×ī³õµÄČŻĘ÷ÖŠøijä1.5mol C”¢4.4mol D£¬ĪĀ¶Č±£³Ö²»±ä£¬ŅŖŹ¹·“Ó¦“ļĘ½ŗāŹ±DµÄÅضČĪŖ0.6mol”¤L£1£¬ŌņČŻĘ÷µÄĢå»żŹĒ””””””””L”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ09-10Äź³¤É³Ķ¬ÉżŗžŹµŃéѧŠ£ø߶žĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»Æѧ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø12·Ö£©t”ꏱ£¬½«3mol
AŗĶ1mol BĘųĢåĶØČėĢå»żĪŖ2LµÄĆܱÕČŻĘ÷ÖŠ£ØČŻ»ż²»±ä£©£¬·¢ÉśČēĻĀ·“Ó¦£ŗ3A£Øg£©+B£Øg£© xC£Øg£©£¬”÷H<0£»ŌŚ2minŹ±·“Ó¦“ļµ½Ę½ŗāדĢ¬£ØĪĀ¶Č²»±ä£©Ź£ÓąĮĖ0.8molB£¬²¢²āµĆCµÄÅضČĪŖ0.4mol”¤L”Ŗ1£¬ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
xC£Øg£©£¬”÷H<0£»ŌŚ2minŹ±·“Ó¦“ļµ½Ę½ŗāדĢ¬£ØĪĀ¶Č²»±ä£©Ź£ÓąĮĖ0.8molB£¬²¢²āµĆCµÄÅضČĪŖ0.4mol”¤L”Ŗ1£¬ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
£Ø1£©“ÓæŖŹ¼·“Ó¦ÖĮ“ļµ½Ę½ŗāדĢ¬£¬Éś³ÉBµÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ ”£
£Ø2£©x= £¬Ę½ŗā³£ŹżK= ”£
£Ø3£©Čō¼ĢŠųĻņŌĘ½ŗā»ģŗĻĪļµÄČŻĘ÷ÖŠĶØČėÉŁĮæŗ¤Ęų£Ø¼ŁÉčŗ¤ĘųŗĶA”¢B”¢C¶¼²»·“Ó¦£©ŗ󣬻ÆŃ§Ę½ŗā£ØĢī×ÖÄø£© ”£
A£®ĻņÕż·“Ó¦·½ĻņŅĘ¶Æ B£®ĻņÄę·“Ó¦·½ĻņŅĘ¶Æ C£®²»ŅʶÆ
£Ø4£©ČōĻņŌĘ½ŗā»ģŗĻĪļµÄČŻĘ÷ÖŠŌŁ³äČėa mol C£¬ŌŚt”ꏱ“ļµ½ŠĀµÄĘ½ŗā£¬“ĖŹ±BµÄĪļÖŹµÄĮæĪŖn£ØB£©= mol”£
£Ø5£©Čē¹ūÉĻŹö·“Ó¦ŌŚĻąĶ¬ĪĀ¶ČŗĶČŻĘ÷ÖŠ½ųŠŠ£¬ÓūŹ¹·“Ó¦“ļµ½Ę½ŗāŹ±CµÄĪļÖŹµÄĮæ·ÖŹżÓėŌĘ½ŗāĻąµČ£¬ĘšŹ¼¼ÓČėµÄČżÖÖĪļÖŹµÄĪļÖŹµÄĮæn£ØA£©”¢n£ØB£©”¢n£ØC£©Ö®¼äÓ¦øĆĀś×ćµÄ¹ŲĻµŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com