
 �Ըߴ�H2ȼ�ϵ����ӽ���Ĥȼ�ϵ�ؾ�������Ч�ʸߡ�����Ⱦ���ŵ㣬��ȼ����������CO���������̵���������Լ״�Ϊԭ����ȡ�ߴ�H2����Ҫ�о�����
�Ըߴ�H2ȼ�ϵ����ӽ���Ĥȼ�ϵ�ؾ�������Ч�ʸߡ�����Ⱦ���ŵ㣬��ȼ����������CO���������̵���������Լ״�Ϊԭ����ȡ�ߴ�H2����Ҫ�о����� ���� ��1���״��ڴ����������ѽ�ɵõ�H2����Ԫ�������ʴ�100%����Ӧ����CO���������õ����������CO�������ȥ��
��2��������ӦΪ���������������ȷ�Ӧ�������¶�������ƽ�������ƶ���������Ӧ���ʣ�
������ˮ���ȣ��״�������������
�ۺ��º����£������ѹǿ֮�ȵ��������ʵ���֮�ȣ�����ƽ��ʱ����������ʵ����������ò���������μӷ�Ӧ�״������ʵ�������������״���ת���ʣ�
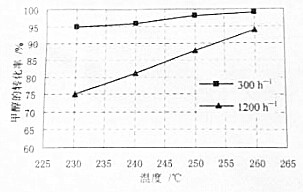
����ͼ��֪������Խ�״���ת�������¶�Ӱ��Խ��
��ͼ��֪��230��ʱ1200h-1��300h-1���ֿ����¼״���ת���ʷֱ�Ϊ75%��95%�������IJ���֮�ȵ��ڲμӷ�Ӧ�״������֮�ȣ�
��3����֪���٣�CH3OH��g��+H2O��g��?CO2��g��+3H2��g������H=+49 kJ•mol-1
�ڣ�CH3OH��g��+$\frac{1}{2}$O2��g��?CO2��g��+2H2��g����H=-193kJ•mol-1
���ݸ�˹���ɣ��١�4+�ڿɵã�5CH3OH��g��+4H2O��g��+$\frac{1}{2}$O2��g��?5CO2��g��+14H2��g����
��� �⣺��1���״��ڴ����������ѽ�ɵõ�H2����Ԫ�������ʴ�100%����Ӧ����CO����������Ӧ����ʽΪ��CH3OH$\frac{\underline{\;����\;}}{\;}$CO+2H2��ȱ���ǵõ����������CO�������ȥ��
�ʴ�Ϊ��CH3OH$\frac{\underline{\;����\;}}{\;}$CO+2H2�����������CO�������ȥ��
��2��������ӦΪ���������������ȷ�Ӧ�������¶�����Ӧ���ʣ�������ƽ�������ƶ����״���ת�������ʴ�Ϊ�������¶ȣ�
������ˮ���ȣ�nH2O��nCH3OH���������ڷ�Ӧ������У���״��������ʣ��ʴ�Ϊ����״��������ʣ�
������ʼ n��H2O��=n��CH3OH��=1mol�����º����£������ѹǿ֮�ȵ��������ʵ���֮�ȣ�ƽ��ʱ����������ʵ���2mol��$\frac{{P}_{2}}{{P}_{1}}$��
CH3OH��g��+H2O��g��?CO2��g��+3H2��g�����ʵ�������
1 2
��$\frac{{P}_{2}}{{P}_{1}}$-1��mol 2mol��$\frac{{P}_{2}}{{P}_{1}}$-2mol=2��$\frac{{P}_{2}}{{P}_{1}}$-1��mol
�ʼ״���ת����Ϊ$\frac{��\frac{{P}_{2}}{{P}_{1}}-1��mol}{1mol}$��100%=��$\frac{{P}_{2}}{{P}_{1}}$-1����100%��
�ʴ�Ϊ����$\frac{{P}_{2}}{{P}_{1}}$-1����100%��
����ͼ��֪������Խ�״���ת�������¶�Ӱ��Խ��
��ͼ��֪��230��ʱ1200h-1��300h-1���ֿ����¼״���ת���ʷֱ�Ϊ75%��95%�������IJ���֮�ȵ��ڲμӷ�Ӧ�״������֮�ȣ�����ͬʱ����H2�IJ�����ǰ��ԼΪ���ߵ�$\frac{1200��75%}{300��95%}$��3.2����
�ʴ�Ϊ����3.2��
��3����֪���٣�CH3OH��g��+H2O��g��?CO2��g��+3H2��g������H=+49 kJ•mol-1
�ڣ�CH3OH��g��+$\frac{1}{2}$O2��g��?CO2��g��+2H2��g����H=-193kJ•mol-1
���ݸ�˹���ɣ��١�4+�ڿɵã�5CH3OH��g��+4H2O��g��+$\frac{1}{2}$O2��g��?5CO2��g��+14H2��g������H=4��49 kJ•mol-1-193kJ•mol-1=+3kJ•mol-1��
�ʴ�Ϊ��+3��
���� ���⿼�黯ѧƽ�������Ӱ�����ء���Ӧ�ȼ���ȣ����ؿ���ѧ����������������ע���˹�����ڷ�Ӧ�ȼ�����Ӧ�ã�


 ��ѧȫ��������ѵ��ϵ�д�
��ѧȫ��������ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | v��A��=1.8 mol•L-1•s-1 | B�� | v��B��=0.3 mol•L-1•s-1 | ||
| C�� | v��C��=0.6 mol•L-1•s-1 | D�� | v��D��=1.6 mol•L-1•s-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��Դ�����Ϻ���Ϣ���ִ���������֧������
��Դ�����Ϻ���Ϣ���ִ���������֧�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
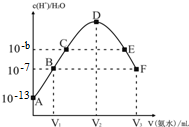
| A�� | E��Һ�д��ڣ�c��NH4+����c��SO42-����c��OH-����c��H+�� | |
| B�� | ϡ�����Ũ��Ϊ0.1mol/L | |
| C�� | C����ҺpH=14-b | |
| D�� | V2=20 mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 5 9 6 | B�� | 5 6 6 | C�� | 3 9 7 | D�� | 4 6 7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| t/min | t0 | t1 | t2 | t3 | t4 | t5 |
| ����NO2�� | $\frac{1}{3}$ | 0.30 | $\frac{4}{15}$ | $\frac{1}{6}$ | $\frac{1}{15}$ | $\frac{1}{15}$ |
| ����SO2�� | $\frac{2}{3}$ | $\frac{19}{30}$ | 0.60 | 0.50 | 0.40 | 0.40 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
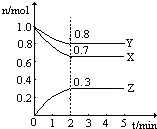 ij�¶�ʱ����һ��2L���ݵ��ܱ������У�X��Y��Z�������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ������ͼ�����ݣ�����д���пհף�
ij�¶�ʱ����һ��2L���ݵ��ܱ������У�X��Y��Z�������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ������ͼ�����ݣ�����д���пհף��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com