
���;�ˮ���������(K2FeO4 )Ϊ����ɫ���壬������ˮ�������Ի�������Һ���ֽ⣬�ڼ�����Һ���ȶ���
)Ϊ����ɫ���壬������ˮ�������Ի�������Һ���ֽ⣬�ڼ�����Һ���ȶ���
��ҵ���Ʊ�K2FeO4�ij��÷��������֡�
��������������������
����������ͼ��ʾ��
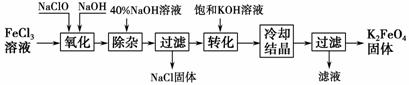
( 1)��ɡ������������з�Ӧ�Ļ�ѧ����ʽ��
1)��ɡ������������з�Ӧ�Ļ�ѧ����ʽ�� 
 ________FeCl3��________NaOH��________NaClO��________Na2FeO4��________ ��________ ��������������________(�ѧʽ)��
________FeCl3��________NaOH��________NaClO��________Na2FeO4��________ ��________ ��������������________(�ѧʽ)��
(2)��ת���������з�����Ӧ�Ļ�ѧ����ʽΪ____________________________
______________________________________________________________��
(3)�������յõ� �ĸ�����س��������ʣ������ؽᾧ���ᴿ�������ǣ����ֲ�Ʒ��________________�ܽ⣬Ȼ��________________
�ĸ�����س��������ʣ������ؽᾧ���ᴿ�������ǣ����ֲ�Ʒ��________________�ܽ⣬Ȼ��________________
������ⷨ��
����Ϊ�����������������Һ��Ȼ��������Һ�м���KOH��
(4)���ʱ����������Ӧ����FeO42���� �õ缫��Ӧ����ʽΪ________________________________________________________________��
�õ缫��Ӧ����ʽΪ________________________________________________________________��
������(1)��Ӧ��NaClO������������ԭ������NaCl������ԭ���غ㣬��֪��Ӧʽ����Ҫ����NaCl��H2O�����ݻ��ϼ���������ƽ��ѧ����ʽΪ2FeCl3��10 NaOH��3NaC lO===2Na2FeO4��9NaCl��5H2O��(2)����(1)�з�Ӧ�Ļ�ѧ����ʽ�͡�ת���������յõ��IJ����֪��ת�����������ڼ���KOH��Һ��Na2FeO4ת��Ϊ�ܽ�ȸ�С��K2FeO4��(3)��ΪK2FeO4�����Ի�������Һ��
lO===2Na2FeO4��9NaCl��5H2O��(2)����(1)�з�Ӧ�Ļ�ѧ����ʽ�͡�ת���������յõ��IJ����֪��ת�����������ڼ���KOH��Һ��Na2FeO4ת��Ϊ�ܽ�ȸ�С��K2FeO4��(3)��ΪK2FeO4�����Ի�������Һ�� �ֽܷ⣬������Ҫ��K2FeO4�ֲ�Ʒ��ϡKOH��Һ���ܽ⣬Ȼ����뱥��KOH��Һ����ȴ�ᾧ��(4)���ʱ��������ǿ���������±�����ΪFeO42����Fe��8OH����6e��===FeO42����4H2O��
�ֽܷ⣬������Ҫ��K2FeO4�ֲ�Ʒ��ϡKOH��Һ���ܽ⣬Ȼ����뱥��KOH��Һ����ȴ�ᾧ��(4)���ʱ��������ǿ���������±�����ΪFeO42����Fe��8OH����6e��===FeO42����4H2O��
�𰸡�(1)2��10��3��2��9��NaCl��5��H2O��NaClO��(2)Na2FeO4��2KOH===K2FeO4��2NaOH��(3)ϡKOH��Һ�����뱥��KOH��Һ����ȴ�ᾧ��(4)Fe��8OH����6e��===FeO42����4H2O


 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ú������������������ڳ��м���ȼ��������ѡ���е���������ڼ���ȼ����Ч�ɷֵ���
A��CO��H2��N2�� ��B.CO��CH4��H2 C.CO��CH4��CO2�� D.CH4��H2��O2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ø�Ч�Ķ�������(ClO2)���Һ�Ƚ����������ɱ�����������彡���к����л��ȴ��
(1)��ҵ�ϣ�ClO2����NaClO3��Na2SO3��Һ��ϲ��������ữ��Ӧ�Ƶã���ѧ��Ӧ����ʽΪ_______________________________________________
_________________________________________________________________��
�ڸ÷�Ӧ��NaClO3��Na2SO3�����ʵ���֮��Ϊ______��
(2)��Ч�Ⱥ����Ǻ�����������һ����Ҫָ�꣬��Ч�Ⱥ����Ķ��壺��HI����������ͬ����I2����Cl2��������ָ������������������֮�ȣ����ðٷ�����ʾ����ClO2����Ч�Ⱥ�����________��
(3)��ѧС���ͬѧ������ClO2������������ˮ������Ԫ�غ����IJⶨ(�ٶ�ClO2ȫ��ת��ΪCl��)�����ǽ���������ʵ�飺��30.00 m Lˮ���мӼ���K2CrO4��Һ��ָʾ������0.001 mol��L��1 AgNO3��Һ�ζ�������ש��ɫAg2CrO4��������ʱ����ζ��յ㣬��ʱ��ȥAgNO3��Һ12.12 mL��
Lˮ���мӼ���K2CrO4��Һ��ָʾ������0.001 mol��L��1 AgNO3��Һ�ζ�������ש��ɫAg2CrO4��������ʱ����ζ��յ㣬��ʱ��ȥAgNO3��Һ12.12 mL��
��ˮ����Cl�������ʵ���Ũ����________��
����֪Ksp(AgCl)��1.780��10��10��Ksp(Ag2CrO4)��2.00��10��12�����ڵζ��յ�ʱ�������Һ��CrO42����Ũ����5.000��10��3 mol��L��1���Լ����ʱ��Һ��Cl����Ũ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ں���Cu(NO3)2��Zn(NO3)2��Fe(NO3)3��AgNO3��0.1 mol�Ļ����Һ�м���0.1 mol���ۣ���ֽ��������ȫ��Ӧ����Һ�в�����Fe3����ͬʱ����0.1 mol Ag�������н����в���ȷ����(����)��
A����Ӧ����Һ��Cu2����Fe2�������ʵ���֮��Ϊ1��2
B�������ԣ�Ag����Fe3����Cu2����Zn2��
C����Fe3������Һ�ɸ�ʴͭ��
D��1 mol Fe�ɻ�ԭ2 mol Fe3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ�У���ȷ���ǣ� ��
A�����Ȼ�������Һ��ͨ������ Fe2++Cl2 Fe3++2Cl-
B������������������ 2Al��6H + 2Al3+��3H2��
C����Ƭ������������Һ��Ӧ�� Al��2OH- AlO2-��H2��
D������ͨ��ˮ�� Cl2+ H2O Cl-+ClO-+2H +
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NO2��ˮ���յķ�Ӧ�У����������뻹ԭ��������ʵ���֮��Ϊ�� ��
A��3��1 B��1��3 C��1��2 D��2��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ӥ���õ�һ������Ƭ����һ������ˮ��ˮ�����ʡ����и߷������п��ܱ����õ��� �� ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1���Ʊ�������������Ļ�ѧ����ʽΪ ��
��2��̼��������ˮ��Һ�еĵ��뷽��ʽΪ ��
��3����֤��Na2SO3��Һ�д���SO32��+H2O HSO3��+OH��ˮ��ƽ�����ʵ�� ����ѡ����ĸ����
HSO3��+OH��ˮ��ƽ�����ʵ�� ����ѡ����ĸ����
A�������̪��Һ��죬�ټ���H2SO4��Һ���ɫ��ȥ
B�������̪��Һ��죬�ټ�����ˮ���ɫ��ȥ
C�������̪��Һ��죬�ټ���BaCl2��Һ����������Һ�ɫ��ȥ
��4�����п��淴Ӧ��2A(g)��2B(g) C(g)��3D(s)�����ܱ��������ݻ����¶ȶ���ͬ�������£��ֱ����������;������ƽ�⣺��. A��B����ʼ���ʵ�����Ϊ2 mol����.C��D����ʼ���ʵ����ֱ�Ϊ2 mol��6 mol������˵������ȷ���� ����ѡ����ĸ����
C(g)��3D(s)�����ܱ��������ݻ����¶ȶ���ͬ�������£��ֱ����������;������ƽ�⣺��. A��B����ʼ���ʵ�����Ϊ2 mol����.C��D����ʼ���ʵ����ֱ�Ϊ2 mol��6 mol������˵������ȷ���� ����ѡ����ĸ����
A��������;�����մﵽƽ��ʱ����ϵ�ڻ������İٷ������ͬ
B���ﵽƽ��ʱ��;�������û�����ܶ�Ϊ;�������û�����ܶȵ�2��
C���ﵽƽ��ʱ��;����C��ƽ��Ũ�ȴ���;����C��ƽ��Ũ�ȵ�2��
��5���ں��ݾ��ȣ�������罻�������������½���2A (g)+ B(g) 2C(g)+ D(s)��Ӧ�����±�����Ͷ�ϣ���Ӧ�ﵽƽ��״̬�������ϵѹǿ���ߡ������÷�Ӧ��ƽ�ⳣ�����¶ȵı仯��ϵ�� ��
2C(g)+ D(s)��Ӧ�����±�����Ͷ�ϣ���Ӧ�ﵽƽ��״̬�������ϵѹǿ���ߡ������÷�Ӧ��ƽ�ⳣ�����¶ȵı仯��ϵ�� ��
| ���� | A | B | C | D |
| ��ʼͶ��/mol | 2 | 1 | 2 | 0 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com