

| (a+0.2a)g |
| 6V |
| 0.2ag |
| V |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��CH4--��Ȼ�� |
| B��CO2--ˮú�� |
| C��CaCO3--ʯ��� |
| D��NaHCO3--�մ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��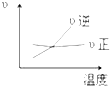 |
B��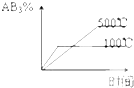 |
C��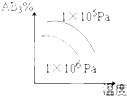 |
D�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 425 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢� | B���٢� | C���ڢ� | D���ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ԭ�����Һ��ֻ����Na+��Fe3+��SO42-�������ܴ���K+��CO32- |
| B����ʵ������ƶ�ԭ�����Һ���Ƿ���SO42- |
| C����ʵ��١��ڿ��ж�ԭ�����Һ���Ƿ���Fe3+ |
| D����ʵ��ۿ��ж�ԭ�����Һ�д���Fe2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
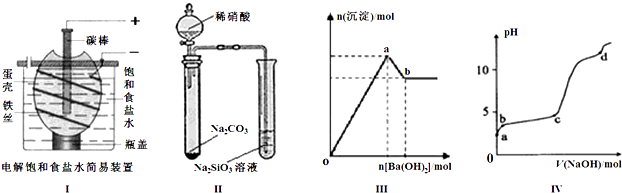
| A����ͼ��װ�õ��һ��ʱ�������������Һ�еμӼ��η�̪����Һ�ʺ�ɫ |
| B����ͼ��װ��ʵ�飬����֤������ǿ����ϵΪ�����̼����� |
| C��ͼ���ʾ����������Һ����μ���Ba��OH��2��Һ�����ɳ��������ʵ�����Ba��OH��2�������ı仯���ߣ���oa�η��������ӷ�ӦΪ�� 2Al3++3SO42-+3Ba2++6OH-=2Al��OH��3��+3BaSO4�� |
| D��ͼ����ʾ������ʱ����1mol?L-1 NaOH��Һ��ε���0.2mol?L-1 Al2��SO4��3��Һ�У�ʵ������ҺpH��NaOH��Һ����仯���ߣ���d��ʱAl��OH��3������ʼ�ܽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���������ʯ��ˮ��ȥNaHCO3��Һ�л��е�����Na2CO3 |
| B�������£�pH=1��ˮ��Һ��NO3-��I-��Na+��Fe3+���Դ������� |
| C���������Һ������þ��Ӧ�����������Ȼ����Һ����þ��Ӧ�������� |
| D��������Ư�۳���������ˮ�ľ�����ɱ�����������ߵ�����ԭ����ͬ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com